Geology Reference
In-Depth Information
extremely important in determining the properties of some
useful minerals.
the electron in its outermost electron shell, leaving its next
shell with eight electrons as the outermost one (Figure 3.5a).
Sodium now has one fewer electron (negative charge) than it
has protons (positive charge), so it is an electrically charged
ion
and is symbolized Na
+
.
The electron lost by sodium is transferred to the outer-
most electron shell of chlorine, which had seven electrons to
begin with. The addition of one more electron gives chlorine
an outermost electron shell of eight electrons, the confi gura-
tion of a noble gas. But its total number of electrons is now 18,
which exceeds by 1 the number of protons. Accordingly, chlo-
rine also becomes an ion, but it is negatively charged (Cl
-
).
An
ionic bond
forms between sodium and chlorine because
of the attractive force between the positively charged sodium
ion and the negatively charged chlorine ion (Figure 3.5a).
In ionic compounds, such as sodium chloride (the min-
eral halite), the ions are arranged in a three-dimensional
framework that results in overall electrical neutrality. In ha-
lite, sodium ions are bonded to chlorine ions on all sides, and
chlorine ions are surrounded by sodium ions (Figure 3.5b).
Ionic Bonding
Notice in Figure 3.2 that most atoms have
fewer than eight electrons in their outermost electron shell.
However, some elements, including neon and argon, have
complete outer shells with eight electrons; because of this
electron confi guration, these elements, known as the
noble
gases
, do not react readily with other elements to form com-
pounds. Interactions among atoms tend to produce electron
confi gurations similar to those of the noble gases. That is,
atoms interact so that their outermost electron shell is fi lled
with eight electrons, unless the fi rst shell (with two electrons)
is also the outermost electron shell, as in helium.
One way that the noble gas confi guration is attained is
by the transfer of one or more electrons from one atom to
another. Common salt is composed of the elements sodium
(Na) and chlorine (Cl); each element is poisonous, but when
combined chemically they form the compound sodium chlo-
ride (NaCl), better known as the mineral halite. Notice in
◗
Figure 3.5a that sodium has 11 protons and 11 electrons;
thus the positive electrical charges of the protons are exactly
balanced by the negative charges of the electrons, and the
atom is electrically neutral. Likewise, chlorine with 17 protons
and 17 electrons is electrically neutral (Figure 3.5a). How-
ever, neither sodium nor chlorine has eight electrons in its
outermost electron shell; sodium has only one, whereas chlo-
rine has seven. To attain a stable confi guration, sodium loses
Covalent Bonding
Covalent bonds
form between atoms
when their electron shells overlap and they share electrons.
For example, atoms of the same element, such as carbon,
cannot bond by transferring electrons from one atom to
another. Carbon (C), which forms the minerals graphite
and diamond, has four electrons in its outermost electron
shell (
◗
Figure 3.6a). If these four electrons were transferred
◗
Figure 3.5
Ionic Bond to Form the Mineral Halite (NaCl)
Cl
-
Na
+
electron transfer
Sodium
atom
11 p
11 e
Chlorine
atom
17 p
17 e
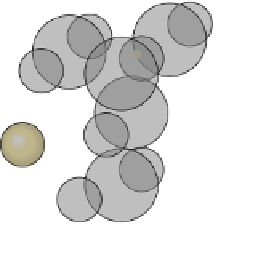
This diagram shows the relative sizes of the
sodium and chlorine atoms and their locations in a
crystal of halite.
b
Sodium
ion
11 p
10 e
Chlorine
ion
17 p
18 e
Image not available due to copyright restrictions
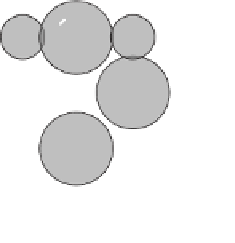
Transfer of the electron in the outermost shell of sodium to the
outermost shell of chlorine. After electron transfer, the sodium and chlorine
atoms are positively and negatively charged ions, respectively.
a















































































































Search WWH ::

Custom Search