Geology Reference
In-Depth Information
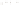
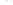
C
Neogene
E
B
A
Paleogene
D
B
E
C
Cretaceous
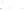
Lingula
A
D
Jurassic
Triassic
Permian
Pennsylvanian
Mississippian
◗
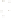
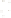
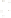
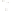
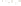
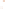
Figure 17.16
Correlation of Two Sections Using Concurrent
Range Zones This concurrent range zone was established by the
overlapping geologic ranges of fossils symbolized here by the letters
A through E. The concurrent range zone is of shorter duration than
any of the individual fossil geologic ranges. Correlating by concurrent
range zones is probably the most accurate method of determining
time equivalence.
Devonian
Atrypa
Silurian
Ordovician
Cambrian
SP
R
Paradoxides
◗
Recording
equipment
in truck
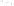
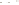
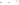
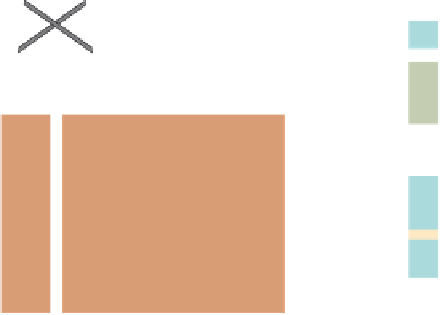
Figure 17.15
Guide Fossils Comparison of the geologic ranges
(heavy vertical lines) of three marine invertebrate animals.
Lingula
is of little use in correlation because it has such a long range. But
Atrypa
and
Paradoxides
are good guide fossils because both are
widespread, easily identifi ed, and have short geologic ranges. Thus,
both can be used to correlate rock units that are widely separated
and to establish the relative age of a rock that contains them.
J.M.R.W
W
ELL
L
OGS
“Let us take the drudgery
out of logging your well”
Radioactive decay
is the process whereby an unstable
atomic nucleus is spontaneously transformed into an atomic
nucleus of a different element. Scientists recognize three
types of radioactive decay, all of which result in a change
of atomic structure (
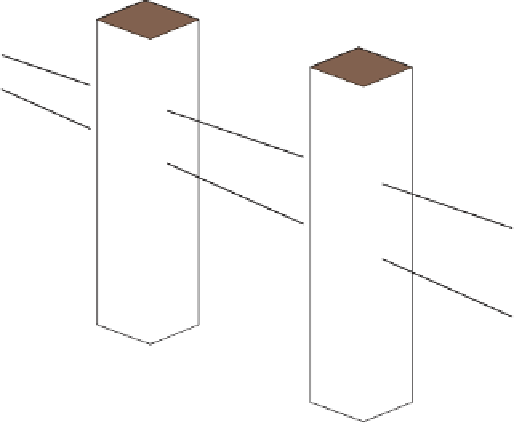
Logging tool
◗
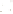
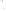
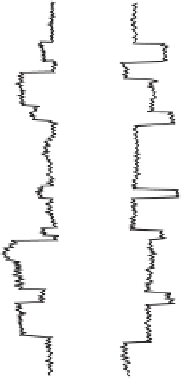
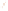
Figure 17.17
Well Logs A schematic diagram showing how well
logs are made. As the logging tool is withdrawn from the drill hole,
data are transmitted to the surface, where they are recorded and
printed as a well log. The curve labeled SP in this diagrammatic
electric log is a plot of self-potential (electrical potential caused
by different conductors in a solution that conducts electricity) with
depth. The curve labeled R is a plot of electrical resistivity with
depth. Electric logs yield information about the rock type and fl uid
content of subsurface formations. Electric logs are also used to
correlate from well to well.
◗
Figure 17.18). In
alpha decay
, 2 pro-
tons and 2 neutrons are emitted from the nucleus, resulting
in the loss of 2 atomic numbers and 4 atomic mass num-
bers. In
beta decay
, a fast-moving electron is emitted from a
neutron in the nucleus, changing that neutron to a proton
and consequently increasing the atomic number by 1, with
no resultant atomic mass number change.
Electron capture
is
when a proton captures an electron from an electron shell
and thereby converts to a neutron, resulting in the loss of
1 atomic number, but not changing the atomic mass number.
Some elements undergo only one decay step in the
conversion from an unstable form to a stable form. For ex-
ample, rubidium 87 decays to strontium 87 by a single beta
emission, and potassium 40 decays to argon 40 by a single
electron capture. Other radioactive elements undergo sev-
eral decay steps. Uranium 235 decays to lead 207 by 7 alpha
and 6 beta steps, whereas uranium 238 decays to lead 206 by
8 alpha and 6 beta steps (
◗
Figure 17.19).



































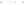



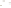


















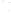



























































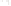


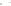




















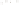



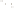


















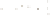





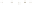



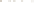

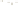
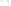




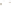



























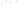



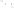
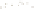
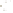












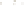
















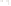






























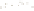
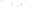













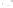






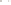


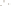

















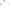



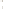








































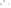



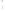


















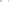

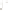





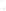
















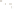








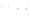
















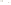
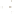



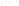





















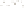
















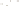







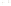























































































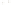





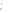
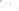



















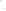








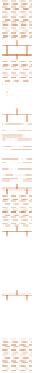







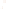




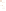

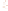

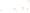





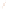












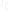


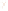


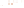







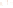
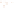









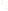
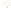










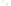

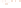




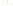

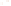








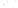


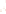







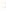






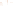










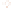


























Search WWH ::

Custom Search