Geology Reference
In-Depth Information
In this reaction, hydrogen ions attack the ions in the
orthoclase structure, and some liberated ions are incorporated
in a developing clay mineral. The potassium and bicarbonate
ions go into solution and combine to form a soluble salt. On
the right side of the equation is excess silica that would not
fi t into the crystal structure of the clay mineral.
Factors That Control the Rate of Chemical Weathering
Chemical weathering operates on the surfaces of particles,
so it alters rocks and minerals from the outside inward. In
fact, if you break open a weathered stone, you will see a
rind of weathering at and near the surface, but the stone
is completely unaltered inside. The rate at which chemi-
cal weathering proceeds depends on several factors. One is
simply the presence or absence of fractures because fl uids
seep along fractures, and weathering is more intense along
these surfaces (
◗
Figure 6.7
Weathering Along Fractures These granitic
rocks in Joshua Tree National Park in California have been
chemically weathered more intensely along fractures than in
unfractured parts of the same rock outcrop.
◗
Figure 6.7). Other factors also control
chemical weathering, including particle size, climate, and
parent material.
Because chemical weathering affects particle surfaces,
the greater the surface area, the more effective the weath-
ering. It is important to realize that small particles have
larger surface areas compared to their volume than do large
particles. Notice in
chemical weathering. The metamorphic rock quartzite is
an extremely stable substance that alters slowly compared
to most other rock types. In contrast, basalt, which con-
tains large amounts of calcium-rich plagioclase and pyrox-
ene minerals, decomposes rapidly because these minerals
are chemically unstable. In fact, the stability of common
minerals is just the opposite of their order of crystallization
in Bowen's reaction series (Table 6.1, also see Figure 4.4):
The minerals that form last in this series are more stable,
whereas those that form early are easily altered because
they are most out of equilibrium with their conditions of
formation.
One manifestation of chemical weathering is
spheroidal
weathering
(
◗
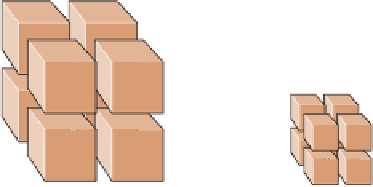
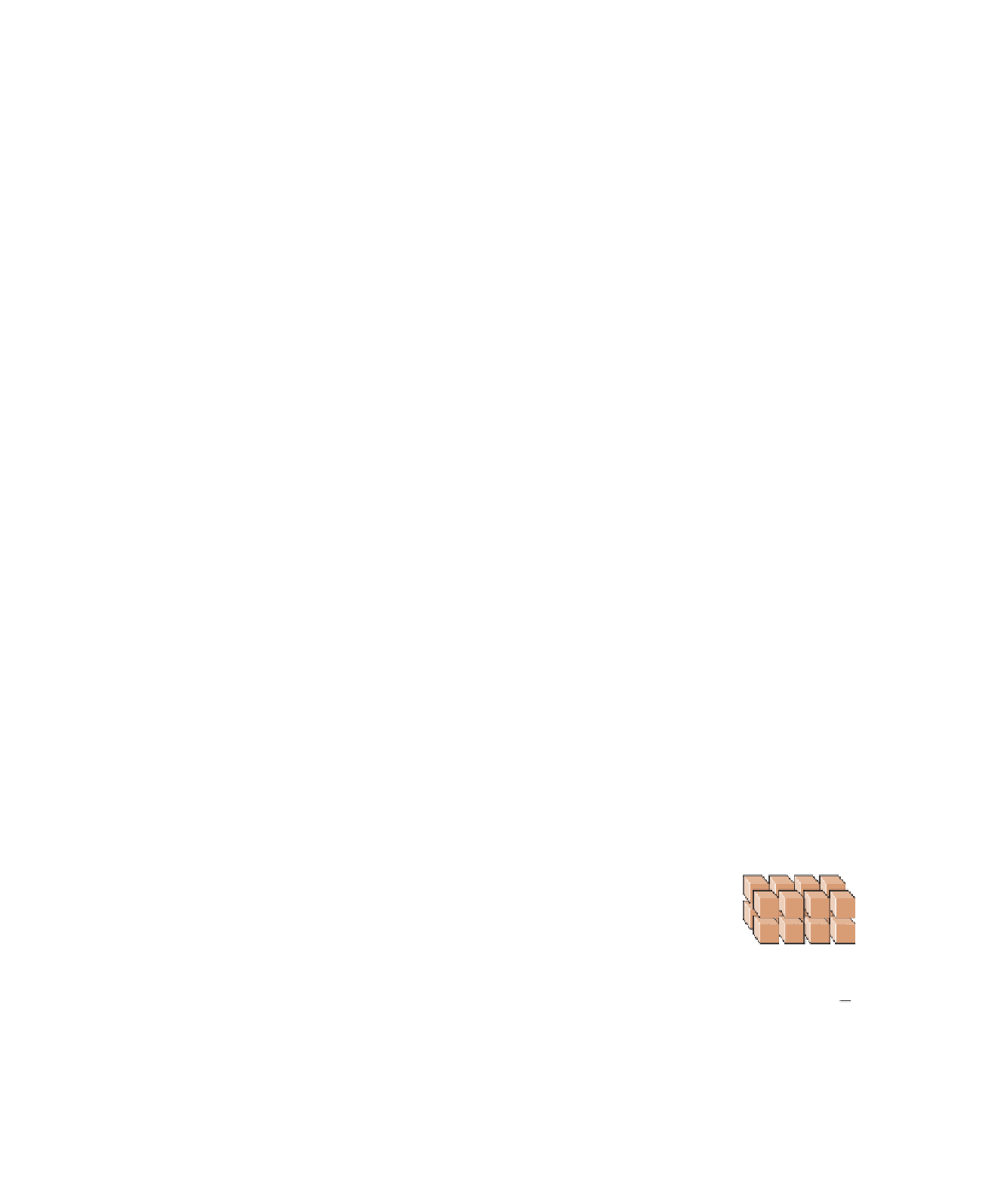
Figure 6.8 that a block measuring 1
m on a side has a total surface area of 6 m
2
, but when the
block is broken into particles measuring 0.5 m on a side,
the total surface area increases to 12 m
2
. And if these par-
ticles are all reduced to 0.25 m on a side, the total surface
area increases to 24 m
2
. Note that although the surface area
in this example increases, the total volume remains the
same at 1 m
3
.
We can conclude that mechanical weathering contrib-
utes to chemical weathering by yielding smaller particles
with greater surface area compared to their volume. Actually,
your own experiences with particle size verify our conten-
tion about surface area and volume. Because of its very small
particle size, powdered sugar gives an intense burst of sweet-
ness as the tiny pieces dissolve rapidly, but otherwise it is the
same as the granular sugar we use
on our cereal or in our coffee.
It is not surprising that chem-
ical weathering is more effective in
the tropics than in arid and arctic
regions because temperatures and
rainfall are high and evaporation
rates are low. In addition, veg-
etation and animal life are much
more abundant. Consequently,
the effects of weathering extend to
depths of several tens of meters,
but they extend only centimeters
to a few meters deep in arid and
arctic regions.
Some rocks are more re-
sistant to chemical alteration
than others, so parent material
is another control on the rate of
Figure 6.9). In spheroidal weathering, a stone,
even one that is rectangular to begin with, weathers to form
a more spherical shape because that is the most stable shape
◗
◗
Figure 6.8
Particle Size and Chemical Weathering
Surface area = 6 m
2
Surface area = 12 m
2
Surface area = 24 m
2
1 m
0.5 m
0.25 m
1 m
0.5 m
0.25 m
b
The surface area is 12 m
2
.
c
The surface area is 24 m
2
,
but the volume remains the
same at 1 m
3
. Small particles
have more surface area in
relation to their volume than do
large particles.
a
As a rock is divided
into smaller particles, its
surface area increases,
but its volume remains the
same. The surface area
is 6 m
2
.




























Search WWH ::

Custom Search