Geology Reference
In-Depth Information
◗
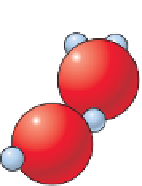
Figure 6.6
The Solution of Halite
104
°
Hydrogen
H
2
O
H
2
O
H
2
O
H
2
O
Cl
-
Oxygen
H
2
O
H
2
O
H
2
O
H
2
O
Na
+
Cl
-
Cl
-
Cumulative
positive
charge
+
Na
+
Cl
-
Hydrogen
Hydrogen
Na
+
H
2
O
Cl
-
Cl
-
H
2
O
Na
+
Cl
-
104
°
Cl
-
Na
+
Na
+
H
2
O
Cl
-
H
2
O
Oxygen
Halite crystal
-
Cumulative
negative
charge
(b)
a
The structure of a water molecule. The asymmetric arrangement
of hydrogen atoms causes the molecule to have a slight positive
electrical charge at its hydrogen end and a slight negative charge at
its oxygen end.
b
Solution of sodium chloride (NaCl), the mineral halite, in water.
Note that the sodium atoms are attracted to the oxygen end of a
water molecule, whereas chloride ions are attracted to the hydrogen
end of the molecule.
hydroxide limonite [FeO(OH)
.
n
H
2
O]. The yellow, brown, and
red colors of many soils and sedimentary rocks are caused by
the presence of small amounts of hematite or limonite.
The chemical reaction between the hydrogen (H
+
) ions and
hydroxyl (OH
-
) ions of water and a mineral's ions is known as
hydrolysis
. In hydrolysis, hydrogen ions actually replace positive
ions in minerals. Such replacement changes the composition of
minerals and liberates iron that then may be oxidized.
The chemical alteration of the potassium feldspar
orthoclase provides a good example of hydrolysis. All feldspars
are framework silicates, but when altered, they yield soluble
salts and clay minerals, such as kaolinite, which are sheet sili-
cates. The chemical weathering of orthoclase by hydrolysis
occurs as follows:
CaCO
3
+ H
2
O + CO
2
m
Ca
++
+ 2HCO
3
-
calcite
water
carbon
calcium bicarbonate
dioxide
ion
ion
The term
oxidation
has a variety of meanings for chem-
ists, but in chemical weathering, it refers to reactions with
oxygen to form an oxide (one or more metallic elements
combined with oxygen) or, if water is present, a hydroxide
(a metallic element or radical combined with OH
-
). For ex-
ample, iron rusts when it combines with oxygen to form the
iron oxide hematite:
4Fe + 3O
2
2Fe
2
O
3
iron oxygen iron oxide
(hematite)
Atmospheric oxygen is abundantly available for oxida-
tion reactions, but oxidation is generally a slow process
unless water is present. Thus, most oxidation is carried out
by oxygen dissolved in water.
Oxidation is important in the alteration of ferromagne-
sian silicates such as olivine, pyroxenes, amphiboles, and bio-
tite. Iron in these minerals combines with oxygen to form the
reddish iron oxide hematite (Fe
2
O
3
) or the yellowish or brown
→
2KAlSi
3
O
8
+ 2H
+
+ 2HCO
3
-
+ H
2
O
→
orthoclase
hydrogen
bicarbonate
water
ion
ion
Al
2
Si
2
O
5
(OH)
4
+ 2K
+
+ 2HCO
3
-
+ 4SiO
2
clay (kaolinite)
potassium
bicarbonate
silica
ion
ion






























































































Search WWH ::

Custom Search