Chemistry Reference
In-Depth Information
(a)
(b)
(c)
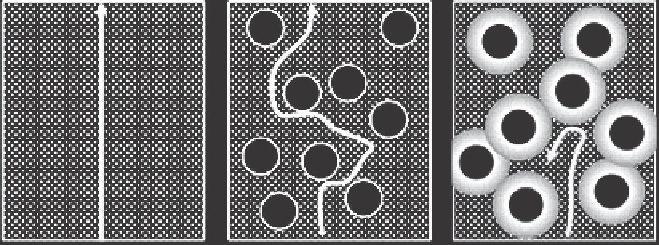
Figure 9.1:
Permeability of a small molecule through PDMS as illustrated for the unfilled polymer
(a), a nonpermeable filler decreasing the permeability of the PDMS by requiring a more
tortuous route around the filler particles (b), and a filler that interacts so strongly with
the PDMS that the PDMS segments close to the filler surface are so restricted that they
are frequently called “bound rubber” (c).
406- 409
surface properties,
64
thermomechanical behavior,
65
optical properties,
66
and
degree of cross linking from the silica-based nanoparticles.
67
Fluorescence
analysis is also possible in silica-PDMS nanocomposites in which the poly-
mer is labeled with dansyl chromophores
68
or lanthanide complexes.
69-71
A variety of other nanoparticles have been formed by such in situ sol-gel
reactions. Examples are the oxides of titanium,
72-79
aluminum,
72,
73
tanta-
lum,
72,
80
zirconium,
73,
77
niobium,
80
and vanadium.
81
Some nanocompos-
ites of this type have also included barium titanate,
82
calcium oxide,
83
calcium salts,
84
borates,
85
HTiNbO
5
,
86
and Eu
3+
dopants.
87
The sol-gel has a number of advantages over the conventional approach
in which separately prepared filler particles are blended into the un-cross-
linked elastomer before vulcanization.
88-90
The time-honored ex situ tech-
nique is difficult to control because the filler particles are generally
agglomerated
91
and the polymer is typically of high enough molecular
weight to make the viscosity of the mixture exceedingly high. Thus, the
blending technique is energy intensive and time consuming, and fre-
quently not entirely successful.
Because of the nature of the in situ precipitation, the particles are es-
sentially unagglomerated (as demonstrated by electron microscopy). The
mechanism for their growth seems to involve simple homogeneous nucle-
ation. Since the particles are separated by polymer, they do not have the
opportunity to coalesce. Figure 9.2 shows a typical transmission electron
micrograph of such a silica-filled material.
92
The particles are relatively
monodisperse, most having diameters in the range of 100-200 Å. Similar
results have been obtained with other particles formed by sol-gel