Environmental Engineering Reference
In-Depth Information
Riccardi et al., 1999; Mbumbia, et al., 2000; Montanaro et al., 2001; Cultrone,
et al., 2004; Lingling et al., 2005). Results from the present study revealed that
by sintering of jarosite-clay CCRs s/s products at 960º±2ºC, the mineralogical
phases of jarosite {KFe
3
(SO
4
)
2
(OH)
6}and
{2Fe
2
O
3
SO
3
.
5H
2
O} changed to
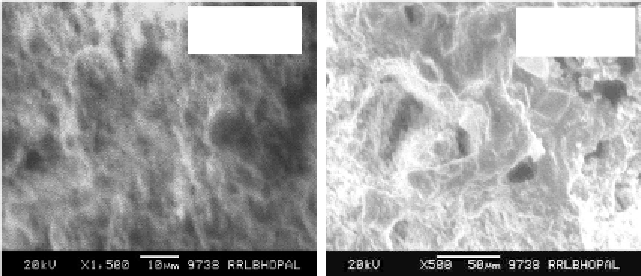
hematite and magnetite. Figure 10 shows SEM microstructure of fracture
surface of sintered jarosite waste composite brick made using jarosite waste to
clay ratio 1 with 15% PCCR.
15% PCCRs
30 % PCCRs
Figure 10. SEM microstructure of fracture surface of sintered jarosite waste composite
brick.
It appears that during firing of jarosite waste stabilized composites, a
considerable amount of liquid phase was formed, which might have reduced
the porosity and specific surface area under the capillary tension forces in the
fine pores resulting less water absorption and higher compressive strength.
This was further verified from the SEM microstructure of the fracture surface
of the jarosite composite bricks as shown in Figure 10 confirming the
densification of the composites which reduced the porosity, water absorption
and increased the density and compressive strength and thus could resist load
for engineering application. More details are reported elsewhere (Asokan,
2004).
3.2.4. Toxicity Leachate Characteristic Study
Earlier studies on s/s of toxic metal wastes using coke and coal
combustion by-products revealed that alkaline wastes can retain low
concentration of toxic metal ions and solidification and sorption of metals
were significant due to the presence of CaO and CaSO
4
in CCRs (Vempati et

Search WWH ::

Custom Search