Environmental Engineering Reference
In-Depth Information
12.0
2.5
10.0
2.0
8.0
1.5
6.0
1.0
4.0
0.5
2.0
0.0
0.0
fiGure 21.21
CO
2
adsorption capacity comparison of hydrothermal-reduced graphene (HTG), mn
3
O
4
, and GmNO samples. Reprinted
with permission from Ref. [82]. © 2012, Springer.
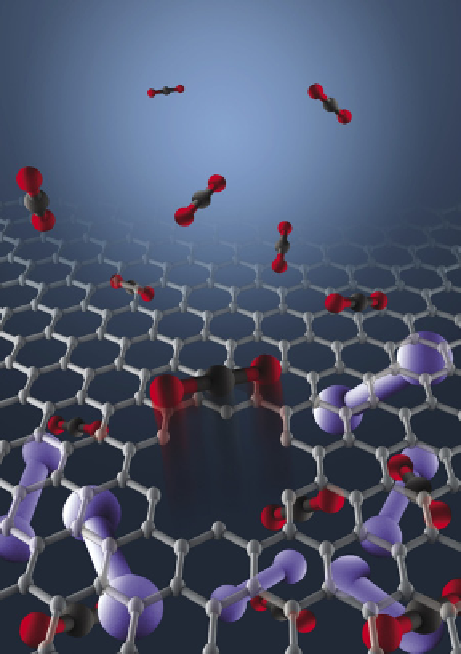
fiGure 21.22
Illustration of a single molecular-sized pore in a graphene membrane separating CO
2
from nitrogen. Image credit: Zhangmin
Huang, University of Colorado, boulder.
It is more difficult to separate CO
2
from a mixture of CO
2
and N
2
(two principal components in a flue gas) because they have
similar kinetic diameters and neither has an electric dipole moment (see Fig. 21.22). It was demonstrated that the chemical
functionalization of graphene can also affect the separation performance of porous graphene membranes. Shan et al. [85]
modeled the effect of the chemical functionalization of the nanopores in a graphene sheet on the separation of N
2
and CO
2
. The
pore rim was simulated with modified structures of all carbons (unmodified pore), all-H passivated, N-H modified, and all-N
modified. The chemical functionalization of the pore rim plays an important role in the selectivity of CO
2
owing to an enhanced
electrostatic interaction. The all-N functionalized pore shows the best CO
2
selectivity (sCO
2
/N
2
= 11).