Environmental Engineering Reference
In-Depth Information
(a)
(b)
(c)
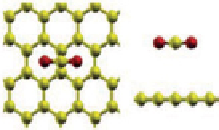
fiGure 21.20
Carbon capture geometry optimized structure: (a) pristine graphene, (b) Ca/graphene at 12.5%, and (c) Ca/graphene at
16.67%. Reprinted with permission from Ref. [80]. © 2011, American Chemical Society.
table 21.3 Gas-adsorption capacities
of Ca/graphene as a function of Ca content
CO
2
uptake
(mmol CO
2
g
-1
sorbent)
Ca content (%)
0
3.48
0-5
3.05
5-15
7.34
15-20
8.91
Adapted from Ref. [80].
Cazorla et al. [80] found that Ca-decorated graphene (Fig. 21.20) possesses unusually large CO
2
uptake capacities in the
range of 2.27-9.09 mmol CO
2
g
-1
sorbent under a low-gas-pressure regime as a result of its unique topology and a strong
interaction between the metal nanoparticles and CO
2
molecules. It is also reported that the interactive strength of the Ca metal
and CO
2
molecule can be efficiently tuned as a function of the Ca loading content (Table 21.3).
Guo et al. [80] confidently proposed that these Ca metal/carbon-based nanomaterial composites exhibit characteristics of
suitable adsorbents for CO
2
capture and sequestration applications.
moreover, Carrillo et al. [81] studied the adsorption of CO
2
on a Ti-graphene system with high metal coverage using the
density functional theory and molecular dynamics simulation. positively charged Ti atoms on graphene surfaces attract nega-
tively charged oxygen atoms toward the graphene surfaces. This force is stronger than the initial repulsion on the C atom by the
Ti atoms. When a CO
2
molecule approaches the graphene surface, it cannot be linear any longer. The O atoms in a bent CO
2
molecule are under different force fields. Thus, one O atom traps an electronic charge from the Ti atoms of the upper plane and
ends up bonded to four Ti atoms, resulting in the CO
2
molecule interacting very strongly with the Ti atoms of the upper plane.
Zhou et al. [82] prepared graphene-mn
3
O
4
(GmNO) materials with high porosity through hydrothermal reaction. They
tested them to the adsorption of CO
2
. Owing to the different contents of mn
3
O
4
, the surface area values of the GmNO samples
vary from about 140 to 685 m
2
g
-1
. It is suggested that the adsorption capacity for the GmNO samples (Fig. 21.21) comes from
the surface area and the basic sites of the mn
3
O
4
nanoparticles.
21.3.1.4.1 Graphene Membranes
Inorganic membranes are much more stable at high temperatures and in the presence of
chemicals than polymeric membranes [83]. Zeolite and silica porous materials can act as molecular sieves, separating gas
(e.g., CO
2
, N
2
, H
2
) molecules by their respective sizes [84]. However, because the sizes of CO
2
, N
2
, H
2
, and other combustion-
specific gases are very similar, membrane porocity must be controlled on a scale comparable to the size differences among
these gas molecules. The separation of gas mixtures using nanoporous materials is an emerging field of research with many
potential applications including fuel cells, batteries, gas sensors, and gas purification. The materials that are currently being
investigated for these applications include organic polymer-based membranes, porous carbon, and inorganic membranes
made of ceramics, metals, or glass, and graphene. Graphene is impermeable to all gases, even to helium, because of the
electron density of its aromatic rings. In order to create a membrane, pores must be created synthetically. Nano-sized pores
have been artificially produced to obtain functional gas membranes using graphene. post-treatment techniques like electron-
hole drilling are suitable for pores in the nanometer range, and smaller pores can be created by catalyzed polymerization at
high temperatures.
Unfortunately, the gas-separation nanomembrane technology is still not at this stage, but active research continues in this
direction.