Environmental Engineering Reference
In-Depth Information
(a)
(b)
24
24
11 bar
9 bar
7 bar
5 bar
3 bar
100°C
50°C
25°C
18
18
12
12
6
6
0
0
0
4
8
P
eq
(bar)
12
0
25
50
75
100
T
(°C)
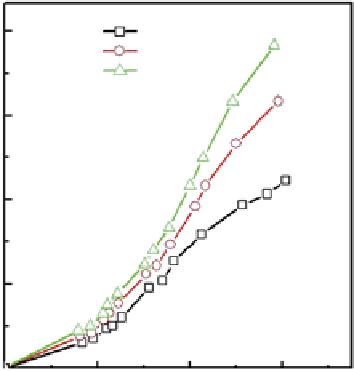
fiGure 21.19
(a) CO
2
adsorption isotherms and (b) temperature variation of adsorption capacity for HEG. Reprinted with permission
from Ref. [68]. © 2011, American Institute of physics.
21.3.1.4 Adsorption of CO
2
in Graphene
Graphene, one of the allotropes (such as CNTs, fullerene, and diamond) of ele-
mental carbon, is a planar monolayer of carbon atoms arranged in a two-dimensional (2D) honeycomb lattice. It could be tai-
lored chemically and structurally in numerous ways: by depositing metal atoms [59] or molecules [60] on top; by incorporating
nitrogen or boron in its structure [61], or by using different substrates to modify the electronic structure [62]. Furthermore, the
main resource for various electrical and optical applications is stemmed due to the interactions between graphene and various
chemical molecules [63]. Graphene, which possesses exceptional mechanical, electrical, thermal, and optical properties, has
numerous potential applications in areas like sensors, solar cells, transistors, and hydrogen storage [64, 65]. As mentioned in
the previous section, CNTs have also been investigated as alternatives for CO
2
adsorption owing to their large surface area and
high porosity [54]. but the cost of CNTs is still prohibitive. Graphene, as a new class of carbon nanomaterials, is found to be
economical and has novel properties similar to CNTs.
The storage capacity of graphene for different gases has been suggested in theoretical studies and CO
2
adsorption
capacity has been demonstrated at very low temperature (195°C), which does not have much practical implication
[66, 67]. mishra and Ramaprabhu [68] suggested the use of graphene oxide for CO
2
adsorption, which can be achieved
by hydrogen-induced thermal exfoliation of graphene oxide at 200°C for possible large-scale production of graphene.
They confirmed the physical adsorption of CO
2
in graphene oxide using Fourier transform infrared spectroscopy. These
authors also reported that maximum adsorption capacities of 21.6, 18, and 12 mmol g
-1
sorbent of hydrogen-exfoliated
graphene (HEG) were observed at 11 bar pressure and temperatures of 25, 50, and 100°C, respectively (see Fig. 21.19).
These values are higher than that of other adsorbents. The adsorption capacity of HEG for CO
2
was found to be higher
than other carbon nanostructures (e.g., activated carbons (ACs), CNTs) and zeolites at the same pressure and tempera-
ture. Siriwardane et al. [69] have reported approximately 7 mmol g
-1
sorbent of CO
2
adsorption in AC. Gensterblum et al.
[70] have reported nearly 6 mmol g
-1
sorbent of CO
2
adsorption in AC at 45°C and 11 bar pressure. Zhang et al. [71] have
reported around 20% enhancement in CO
2
uptake by modifying the AC with nitrogen at room temperature and have
shown around 16 mmol g
-1
sorbent adsorption capacity at 11 bar. Cavenati et al. [72] have reported approximately
3.2 mmol g
-1
of CO
2
adsorption in 13x zeolite at 12 bar pressure and room temperature. A high-pressure CO
2
adsorption
study on different mOFs by millward and Yaghi exhibits CO
2
adsorption capacity ranging from 2 to 8 mmol g
-1
under
similar conditions [30].
Inorganic nanoparticles, that is, metal [73] and metal oxides [74], have been successfully incorporated between graphene
sheets, and their performance has been investigated in a wide variety of applications in the fields of catalysis [75], gas sorption
[76], and electrode materials [77]. The incorporation of metal atoms and oxides over the surface area of graphene shows high
CO
2
uptake volume. meanwhile, nanoparticles incorporated between graphene sheets effectively prevent the aggregation of
graphene-nanoparticle composites in the hybrid system [78, 79], and their high porosity could increase their performance as an
adsorbent and in other applications.