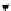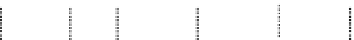Environmental Engineering Reference
In-Depth Information
(g)
(h)
-10
1.10
Fe
Fe + Cu + Al
-12
-14
1.05
-16
-18
1.00
-20
Fe + Cu
-22
0.95
-24
-26
0.90
0
10
20
30
40
50
60
0
10
20
30
40
50
60
Days in operation
Days in operation
(i)
(j)
20
10
18
8
16
6
14
4
12
2
10
0
8
-2
6
-4
4
-6
2
-8
0
-10
0
10
20
30
40
50
60
0
20
40
60
Days in operation
Days in operation
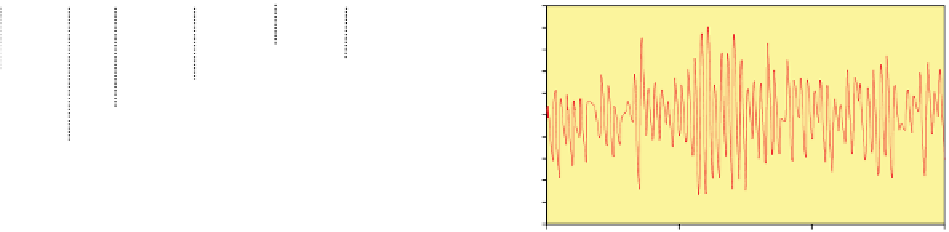
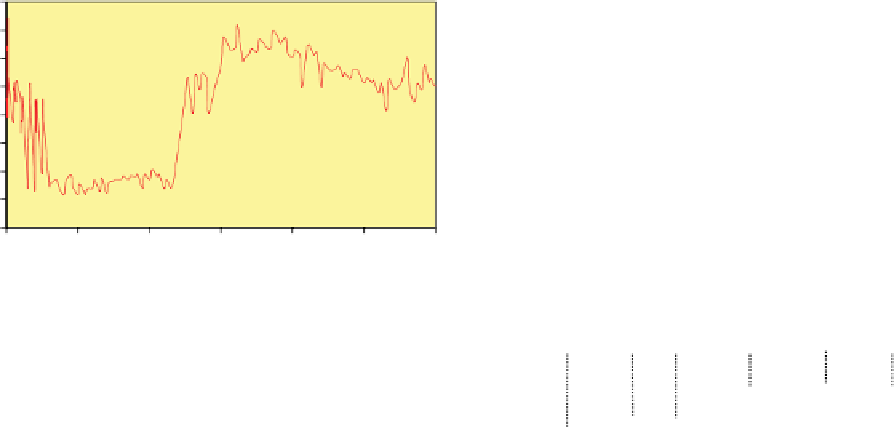
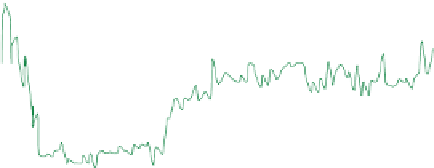
fiGure 1.1
(
Continued
) (g)
p
H
2
vs. time in a static diffusion reactor for nano-ZVM (Fig. 1.1e and f). (h) Typical declining EC oscillations
with time. n-Fe
0
[135]. (i) Variation in temperature with time (Figs. 1.1e-h) (j) Temperature oscillations with time.
1. Pollutants of the form MO
x
, where M = S, n, C, P, Cl (e.g., nitrates [24-30], nitrites [30-33], perchlorates [34-37],
carbonic acids [38-45], phosphates [46], sulfates [47-50]). Pollutants are removed within green rust, or ZVM-hydroxide/
peroxide structures, or polyoxometallate (POM) structures, by cation/anion substitution, or by layer accretion [38-50].
Green rusts are highly reactive structures comprising [38-50] alternating positively and negatively charged hydroxide/
peroxide layers and hydrated anion layers with the general composition [Cation
a
I
Cation
c
II
Cation
b
III
(OH)
m
(OOH)
d
]
x
+
[(
A
y
/
nv
yiH
2
O]
x
−
. Cations and anions can be substituted [38-50].
A
is an anion (e.g., Cl
−
, SO
4
2−
, CO
3
2−
, Br
−
, I
−
, nO
3
−
,
ClO
4
−
, SO
3
2−
, SeO
4
2−
, PO
4
2−
, OH
−
, OOH
−
, O
x
y
−
, etc.); nv = valency; yi = the inter-layer water and is typically between 2
and 4. A typical green rust forms as plates 5-2000 nm in diameter and about 40 nm thick, for example, [50]. Green rusts
(ZVM degradation products) are highly efficient anion and cation scavengers and may be as reactive, or more reactive,
than Fe
0
[9, 49]. During scavenging operations, the “green rusts” can incorporate cation layers of the form [Cation
e
I
(OH)
m
(OOH)
d
]
x
+
and [Cation
f
IV
(OH)
m
(OOH)
d
]
x
+
and higher valent cation hydroxides/peroxides.
2. Gases, including H
2
S [51], O
2
[52], CO
2
[53], CO [53], H
2
[53].
3. Halogenated ions of the form [halogen]
x
O
y
(e.g., chlorates, bromates, perchlorates, etc.), and C
x
[Halogen]
y
O
z
[34-37] and
halogenated organic compounds of the general form C
x
H
y
[Halogen]
z
, where
y
can be 0. The halogen is one or more of Cl,
Br, I, F. [54-57], for example, chloromethane (CM), trichloromethane (TCM), dichloromethane (DCM), tetrachloro-
methane; perchloroethylene (PCE), trichloroethylene (TCE), dichloroethylene (DCE); vinyl chloride (VC); hexachloro-
ethane, tetrachloroethane, trichloroethane, dichloroethane, chloropropane (etc.), chlorobutane (etc.), chlorobenzene,
(etc.), ethylene dibromide (EDB), perchlorate, polychlorinated biphenyls (PCB's). The end degradation products take the
generic form C
x
H
y
(e.g., methane, ethyne, ethene, ethane, propane, butane, pentane, hexane, heptane, octane). These may
be further altered to form products of the form: H
x
C
y
O
z
or ring structures.
4. Organic peroxides (e.g., triacetone triperoxide (TATP))[58].
5. Organic nitrogenous compounds, including azo dyes [59-61], atrazine [62, 63], cyclonite/hexogen (RDX) [14, 64],
dinitrotoluene (DnT) [65, 66], nitrosodimethylamine (nDMA) [67, 68], nitrocellulose [69], tetramethylenetetranitra-
mine (HMX) [70-72], trinitrotoluene (TnT) [73-75], disinfection by-products (DBPs) [76, 77], fertilizers [78, 79],
pesticides [80-83], herbicides [84, 85], fungicides [86].