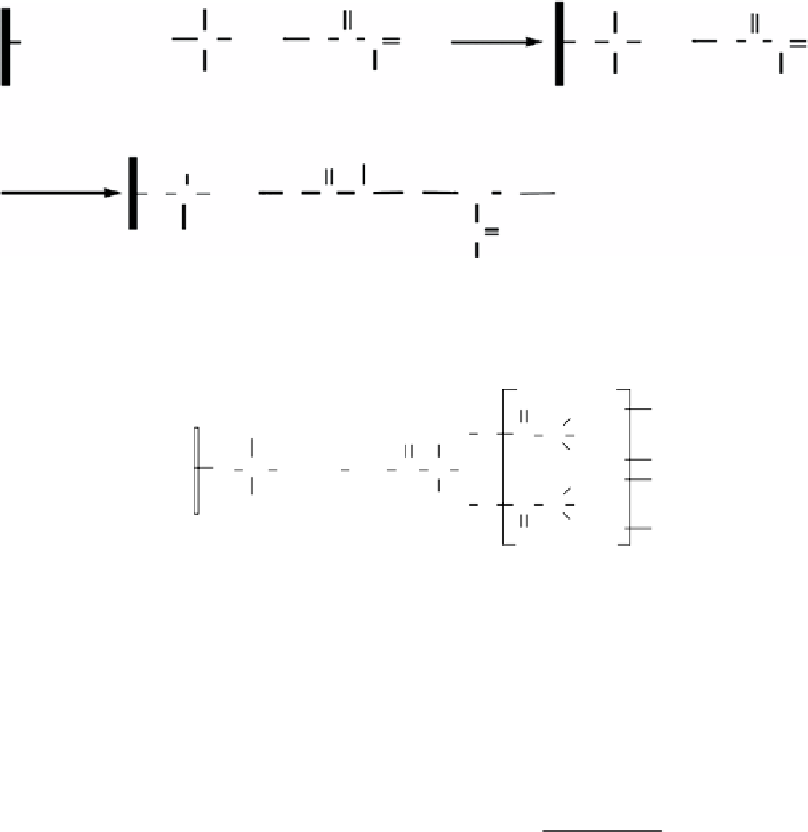Environmental Engineering Reference
In-Depth Information
O
O
Toluene
O
CC CH
2
OH + (CH
3
OH)
3
Si
(CH
3
)
3
(CH
3
)
3
O
CC CH
2
OSi
CH
3
CH
3
AT P
CH
3
O
H
2
H
2
AIBN
Acrylamide
[
]
O
Si
O
CC
C
CH
C
(CH
3
)
3
n
H
O
C
NH
2
PAM/ATP
figure 16.14
Preparation procedure of PAm/ATP.
O
CH
2
O
O
CH
2
O
CC
CH
3
CH
2
O
CH
2
O
OSi
(CH
2
)
3
NH
CC
CH
3
CH
2
O
C
C
CH
3
CH
2
O
O
n
figure 16.15
Ideal structure of the HAPe-ATP.
table 16.2
Competitive adsorption data of atP adsorbents
Adsorption capacities (mg/g)
samples
Cu(II)
Hg(II)
Zn(II)
Cd(II)
Bare ATP
0.41
0.32
0.97
0.37
A-ATP
2.45
5.29
0.18
0.34
HAPe-ATP
1.76
3.52
0.45
0.29
(fig. 16.15) [93]. The competitive adsorption capacities of the three clay adsorbents, bare ATP, aminopropyl ATP (A-ATP), and
HAPe-grafted ATP (HAPe-ATP), toward the four HmI (Cu(II), Hg(II), Zn(II), and Cd(II)), were also compared (Table 16.2).
It was found that bare ATP had higher adsorption capacities for Zn(II) and Cd(II) ions. However, the A-ATP and the HAPe-
ATP adsorbents had higher adsorption capacities for Cu(II) and Hg(II) ions. furthermore, the adsorption capacities for Cu(II)
and Hg(II) ions of the HAPe-ATP clay adsorbent were slightly lower than those of the A-ATP clay adsorbent because the
groups containing the n element were enshrouded with HAPe, which is not soluble in water, and the ester and hydroxyl groups
of HAPe had lower complexation abilities toward these HmI. However, HAPe-ATP had a faster sedimentating rate than
A-ATP. This is an advantage in practical applications.
The organic-inorganic hybrid of poly(acrylic acid-acrylonitrile)/ATP, P(A-n)/AT nanocomposites, was prepared by in situ
polymerization and composition of acrylic acid (AA) and acrylonitrile (An) onto modified ATP nanoparticles (fig. 16.16) [94].
The resultant P(A-n)/AT nanocomposites were transformed into a novel nanoadsorbent of poly(AA-acryloamidoxime)/ATP by
further functionalization, that is, P(A-O)/AT nanoadsorbent. The adsorption properties of P(A-O)/AT toward metal ions were
determined, and the results indicated that adsorbents with nanocomposite structure had good selectivity toward Pb
2+
among the
numerous metal ions. The maximum removal capacity of Pb
2+
ions was up to 109.9 mg/g, and it is notable to see that the adsorp-
tion removal of the P(A-O)/AT nanoadsorbent for Pb
2+
ions could achieve more than 96.6% when the initial concentration of
Pb
2+
ions was 120.0 mg/l. The kinetics, isotherm models, and conductivity indicated that it could be a chemisorption process
and the best coordination took place when AO:AA:Pb
2+
= 1:1:1. In addition, after treatment with cetyltrimethylammonium
bromide (CTAB), the P(A-O)/AT nanoadsorbent showed better adsorption properties for phenol than similar kinds of
materials.