Environmental Engineering Reference
In-Depth Information
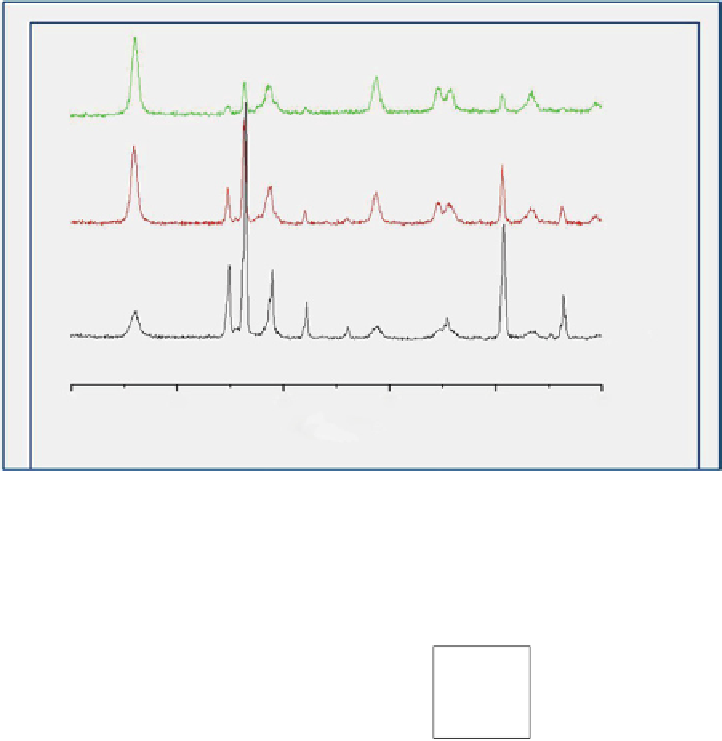
SG80
SG50
SG20
20
30
40
50
60
70
2 θ
figure 12.10
X-ray diffraction (XRd) patterns of SiC-TiO
2
samples prepared via the sol-gel method.
1.1
1
P-25
SG20
sg50
sg80
0.9
0.8
0.7
0.6
0.5
0.4
0
20
40
60
80
100
Time (min)
120
140
160
180
200
figure 12.11
Methylene blue degradation using SiC-TiO
2
compounds prepared via the sol-gel method.
In this case, it was observed that this condition induces a synergistic effect between the two components of the catalyst that
reduces the recombination of the electron-hole pairs , favoring the photocatalytic activity.
In Figure 12.11, the photocatalytic curves for methylene blue degradation are shown; it is observed that SiC-TiO
2
samples
have better degradation capacity than TiO
2
.
The kinetic parameters for this reaction are showed in Table 12.6. Although half-life reaches almost 200 min, all SiC-TiO
2
compounds are more active than TiO
2
.
The degradation of indigo carmine occurred faster than that of methylene blue. This is because the nature of the dye allows
easy degradation (Fig. 12.12).
Table 12.7 shows the kinetic parameters for indigo carmine photodegradation. The half-life in this case is lower than the
half-life for methylene blue. In fact, total degradation of indigo carmine is achieved in less than 1 h.
As mentioned, the catalysts discussed here were designed and prepared with specific structural, physicochemical, and
electronic characteristics in order to be considered as active materials in photoinduced processes.