Environmental Engineering Reference
In-Depth Information
table 12.6 Kinetic parameters of methylene blue
degradation using Sic-tio
2
as photocatalyst
Material
k
(min
−1
)
t
1/2
(min)
TiO
2
P-25
0.0024
289
SG20
0.0027
257
SG50
0.0025
277
SG80
0.0031
224
1.2
P-25
sg20
1
sg50
sg80
0.8
0.6
0.4
0.2
0
0
20
40
60
80
100
Time (min)
120
140
160
180
200
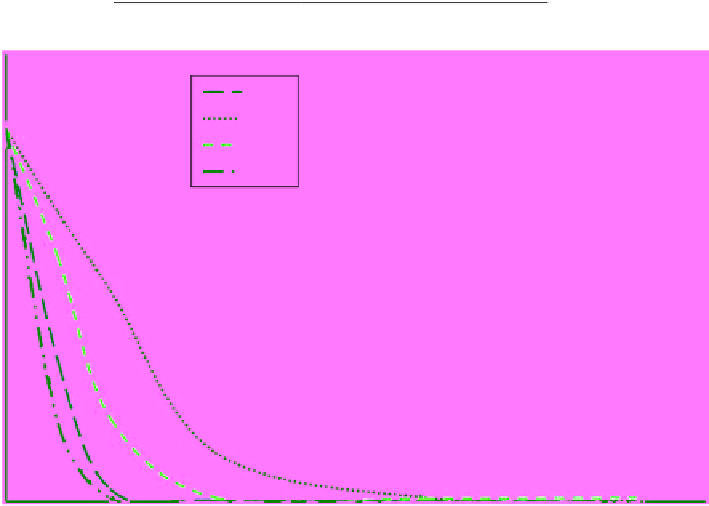
figure 12.12
Indigo carmine degradation using SiC-TiO
2
compounds prepared via the sol-gel method.
table 12.7 Kinetic parameters of indigo carmine degradation
using Sic-tio
2
as photocatalyst
Material
k
(min
−1
)
t
1/2
(min)
TiO
2
P-25
0.0154
45
SG20
0.0145
48
SG50
0.0165
42
SG80
0.0331
21
In some cases, these catalysts presented photocatalytic performance better than or similar to TiO
2
, particularly due to the
different methods of preparation like sol-gel, which allows the control of morphology, surface area, and porosity enhancement.
Additionally, the presence of metal transition cations as dopant agents modified the band gap value in order to activate the cat-
alysts under UV and visible light irradiation. In this sense a relationship was established between the performance-structure
and the synthesis route of several catalyst materials.
acKnowledgmentS
The authors thank CONACyT for the financial support obtained through projects CB168730-2011, CB177099-2012, INFR03-
2011-173625 and INFRA-2012-01-187552, CNPq Mexico-Brasil 174247, and FOINS/75/2012. Thanks to PAICyT-
UANL-2012 projects and PIFI-2011. Additionally, the authors thank Christian Gomez-Solis, Ph.d., and Miguel A. Ruiz-Gomez,
Ph.d., for their invaluable collaboration.