Environmental Engineering Reference
In-Depth Information
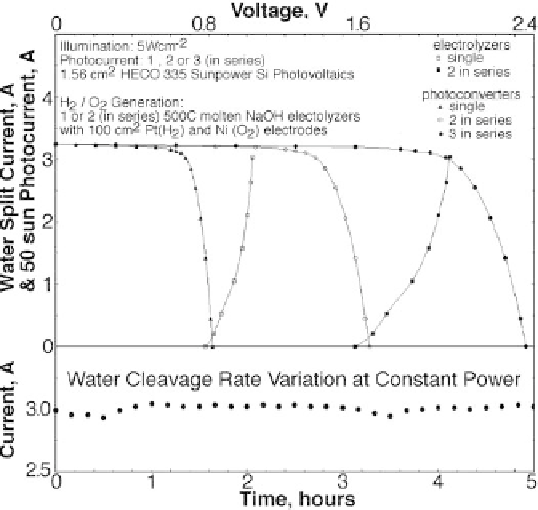
Figure 8.3.2
Photovoltaic and electrolysis charge transfer of STEP hydrogen using Si CPV's driving
molten NaOH water electrolysis. Photocurrent is shown for 1, 2 or 3 1.561 cm
2
HECO
335 Sunpower Si photovoltaics in series at 50 suns. The CPV's drive 500
◦
C molten
NaOH steam electrolysis using Pt gauze electrodes. Left inset: electrolysis current stability.
Modified with permission from Licht 2011.
Wang, 2006). Higher conductivity is desired as it leads to lower electrolysis ohmic
losses. Low carbonate melting points are achieved by a eutectic mix of alkali carbon-
ates (T
mp
Li
1
.
07
Na
0
.
93
CO
3
: 499
◦
C; Li
0
.
85
Na
0
.
61
K
0
.
54
CO
3
: 393
◦
C). Mass transport is
also improved at higher temperature; the conductivity increases from 0.9 to 2.1 S cm
−
1
with temperature increase from 650
◦
Cto875
◦
C for a 1:1:1 by mass mixture of the
three alkali carbonates (Kojima et al., 2008).
In 2009 we showed that molten carbonate electrolyzers can provide an effec-
tive media for solar splitting of CO
2
at high conversion efficiency. In 2010 Kaplan,
et al., and our group separately reported that molten lithiated carbonates provide a
particularly effective medium for the electrolytsis reduction of carbon dioxide (Licht
et al., 2010a; Kaplan et al., 2010). As we show in the photograph in Figure 8.3.3,
at 750
◦
C, carbon dioxide is captured in molten lithium carbonate electrolyte as solid
carbon by reduction at the cathode at low electrolysis potential. It is seen in the cyclic
voltammetry, CV, that a solid carbon peak that is observed at 750
◦
C is not evident
at 950
◦
C. At temperatures less than
900
◦
C in the molten electrolyte, solid carbon
is the preferred CO
2
splitting product, while carbon monoxide is the preferred prod-
uct at higher temperature. As seen in the main portion of the figure, the electrolysis
potential is
<
1.2 V at either 0.1 or 0.5 A/cm
2
, respectively at 750 or 850
◦
C. Hence,
∼
Search WWH ::

Custom Search