Environmental Engineering Reference
In-Depth Information
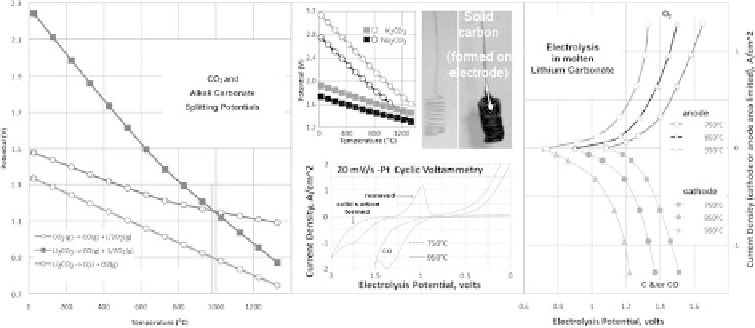
Figure 8.3.3
The calculated (left) and measured (right) electrolysis of CO
2
in molten carbonate. Left:
The calculated thermodynamic electrolysis potential for carbon capture and conversion
in Li
2
CO
3
(main figure), or Na
2
CO
3
or K
2
CO
3
(left middle); squares refer to M
2
CO
3
to
C
+
M
2
O
+
O
2
and circles to a M
2
CO
3
to CO
+
M
2
O
+
1/2O
2
. To the left of the vertical
brown line, solid carbon is the thermodynamically preferred (lower energy) product. To
the right of the vertical line, CO is preferred. Carbon dioxide fed into the electrolysis
chamber is converted to solid carbon in a single step. Photographs: coiled platinum cathode
before (left), and after (right), CO
2
splitting to solid carbon at 750
◦
C in molten carbonate
with a Ni anode. Right: The electrolysis full cell potential is measured, under anode or
cathode limiting conditions, at a platinum electrode for a range of stable anodic and
cathodic current densitites in molten Li
2
CO
3
. Lower midde: cathode size restricted full
cell cyclic voltammetry, CV, of Pt electrodes in molten Li
2
CO
3
. Modified with permission
from Licht et al. 2010a.
the electrolysis energy required at these elevated, molten temperatures is less than the
minimum energy required to split CO
2
to CO at 25
◦
C:
E
◦
(T
25
◦
C)
CO
2
→
CO
+
1
/
2O
2
=
=
1
.
33 V
(8.3.1)
The observed experimental carbon capture correlates with:
Li
2
CO
3
(molten)
→
C (solid)
+
Li
2
O (dissolved)
+
O
2
(gas)
(8.3.2A)
Li
2
CO
3
(molten)
→
CO (gas)
+
Li
2
O (dissolved)
+
1
/
2O
2
(gas)
(8.3.2B)
When CO
2
is bubbled in, a rapid reaction back to the original lithium carbonate is
strongly favored:
Li
2
O (dissolved)
+
CO
2
(gas)
→
Li
2
CO
3
(molten)
(8.3.3A)
Li
2
CO
3
→
Li
2
O
+
CO
2
(8.3.3B)
In the presence of carbon dioxide, reaction (8.3.3A) is strongly favored (exothermic),
and the rapid reaction back to the original lithium carbonate occurs while CO
2
is
bubbled into molten lithium carbonate containing the lithium oxide.
Search WWH ::

Custom Search