Environmental Engineering Reference
In-Depth Information
10
0.01
9
1
8
100
7
6
5
4
Ty p e I I
3
2
Ty p e I I I
1
0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
y
i
FIGURE 3.18
Different shapes of the BET isotherm. Note that the shapes vary with the
isotherm constant,
K
B
.
equation can be recast into the following form:
1
Γ
i
·
ψ
i
1
K
BET
Γ
(K
BET
−
1
)
− ψ
i
=
i
+
· ψ
i
,
(3.83)
m
1
K
BET
m
so that both
K
BET
and
i
can be obtained from the slope and intercept of a linear
regression of the LHS versus
y
i
. The linear region of the plot typically lies between
ψ
i
values of 0.05 and 0.3 and extrapolation below or above this limit should be
approached with caution. If the specific area of the adsorbate,
Γ
σ
m
, is known, then the
total surface area of the adsorbent can be obtained from the equation
m
i
σ
m
N
Γ
=
S
a
.
(3.84)
Pure nitrogen, argon, or butane with specific surface areas of 16.2, 13.8, and 18.1
×
10
−
16
cm
2
/molecule are used for this purpose.
E
XAMPLE
3.19 U
SE OF THE
B
ET
E
QUATION
Poe et al. (1988) reported that the vapor adsorption of an organic compound (ethyl ether)
on a typical dry soil (Weller soil fromArkansas) showed distinct multi-layer formation.
The following data were obtained:
continued








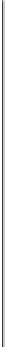









Search WWH ::

Custom Search