Environmental Engineering Reference
In-Depth Information
Solute in the bulk phase
n
=
∞
Multilayers
Monolayer
n
=
2
n
=
1
Solid surface
FIGURE 3.17
A schematic of the adsorption of a compound from either the gas phase or
liquid phase on to a solid. Simultaneous formation of mono- and multilayers of solute on the
surface is shown. Note that the same phenomenon can also occur on a liquid surface instead of
the solid surface. The BET isotherm applies in either case.
n
=
2, 3, 4,
...
, is the same, but is different for a molecule in the layer
n
=
1, one
obtains
θ
i
=
Γ
i
Γ
K
BET
ψ
i
i
=
,
(3.82)
m
(
1
− ψ
i
)
{
1
+
(K
BET
−
1
)
· ψ
i
}
m
i
P
i
/P
i
where
Γ
is the monolayer capacity and
ψ
i
=
for adsorption from the gas
C
i
/C
i
for adsorption from the liquid phase.
P
i
phase and
ψ
i
=
is the saturation
vapor pressure of solute
i
in the gas phase, whereas
C
i
is the saturated concen-
θ
i
can now be greater than 1,
indicating multi-layer adsorption.
K
BET
is the BET adsorption constant. Figure 3.18
illustrates the isotherm shapes for different values of
K
BET
. For values of 10 and
100, a clear transition from a region of monolayer saturation coverage to multi-layer
is evident. These isotherms are characterized as Type II. The Langmuir isotherm
is characterized as a Type I isotherm. When the value of
K
BET
is very small (0.1
and 1 in Figure 3.18), there is no such clear transition from monolayer to multi-
layer coverage. These isotherms are called Type III isotherms. There are numerous
examples in environmental engineering where Type II isotherms have been observed;
examples include the adsorption of volatile organic compounds (VOCs) on soils
(Chiou and Shoup, 1985; Valsaraj and Thibodeaux, 1988) and aerosol particulates
(Thibodeaux et al., 1991). Type III isotherms are also common in environmental
engineering, for example, the adsorption of hydrocarbon vapors on water surfaces
(Hartkopf and Karger, 1973; Valsaraj, 1988) and in soil-water systems (Pennel et al.,
1992).
The BET isotherm is often used to obtain the surface areas of soils, sediments, and
other solid surfaces by monitoring the adsorption of nitrogen and other inert gases.
The method has also enjoyed use in the environmental engineering area. The BET
tration of solute
i
in the liquid phase. Note that



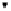











Search WWH ::

Custom Search