Environmental Engineering Reference
In-Depth Information
arsenic concentrations under constant solution low rate and amount of adsorbent. For
each case, the solute uptake determined by the area above the breakthrough curve was
found to be within 10% of corresponding equilibrium loading, suggesting the almost com-
plete utilization of the adsorbent under low conditions.
36.9 Carbon Micro-/Nanoparticles as Adsorbents for Arsenic
It was mentioned in Section 36.4 that the novelty of such carbon micro-/nanoparticles is
the
in situ
incorporation of Fe in their polymeric matrix during the polymerization stage.
Furthermore, the carbon beads (~0.8 mm) produced after carbonization and activation of
the micron-sized beads contained uniform and high loading (~2.5% w/w) of Fe particles.
The carbon nanoparticles (~100 nm) produced after ball milling of the polymeric beads,
followed by carbonization and activation, retained the Fe-nanoparticles and were found
to have even higher adsorption capacity than the micron-sized particles. Another salient
feature of these adsorbents is that the maximum removal of As(III) or As(V) occurs at pH
between 6.5 and 7.5. Moreover, the variation in pH of the solution increased marginally
(not more than 20%) during adsorption. Considering that pH of ground or potable water
is commonly in the vicinity of 7 (usually between 6 and 7.5), the maximum adsorption of
arsenic at pH ~7 and marginal variation in pH observed during adsorption suggest that
pretreatment or posttreatment of water may not be required using these adsorbents.
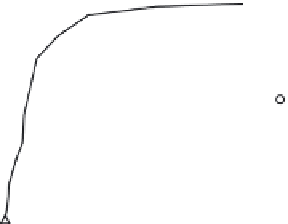
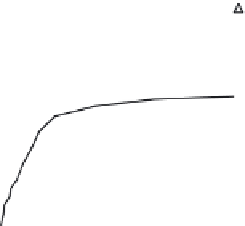
Figure 36.13 presents the equilibrium concentration of arsenic ions (As(III) and As(V))
in the solid phase as a function of the aqueous-phase concentration for four types of
samples: Fe-doped phenolic beads (PBFe), carbonized/activated beads (PBFe-Act), and
Fe-incorporated carbon nanoparticles produced before and after the carbonization step
(PBFe-Act-BM and PBFe-BM-Act, respectively). The lines regressed through the data are
essentially the equilibrium isotherms at 30°C over the aqueous-phase concentration range
of 0.1-10 mg/l or ppm. The saturation capacity of PBFe-BM-Act for As(III) is approximately
13 mg/g, while for As(V) it is 5 mg/g. Revisiting Section 36.8, these values are considerably
(a)
(b)
6
14
As(V)
As(III)
12
5
10
PBFe-BM-Act
PBFe-Act-BM
PBFe-Act
PBFe
4
8
3
6
PBFe-BM-Act
PBFe-Act-BM
PBFe-Act
PBFe
2
4
1
2
0
0
0
1
2
3
4
5
6
7
0
1
2
3
4
5
6
Aqueous-phase concentration (mg/l)
Aqueous-phase concentration (mg/l)
FIGURE 36.13
Adsorption equilibrium isotherms for various Fe-impregnated adsorbents (
T
= 303 K, pH 6.5 for As(V) (a) and
pH 7 for As(III) (b)). (From A.K. Sharma et al.,
Chem. Eng. Sci
., 65, 3591, 2010.)