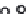Environmental Engineering Reference
In-Depth Information
higher than the reported literature data in most cases and comparable in a few cases, for
both As(III) and As(V). As observed in Figure 36.13, adsorption of arsenic ions by PBFe-
BM-Act is signiicantly larger than that by the other adsorbents. Additionally, the adsorp-
tion of PBFe-Act-BM is shown to be approximately 10%-15% higher than that of PBFe-Act,
implying the milling of activated beads results in a small increase of the adsorption capac-
ity. These results are also consistent with the BET surface area and pore volumes of the
various samples presented in Table 36.2.
Similar to the column studies carried out for the nanoiber-based adsorbents, the perfor-
mance of the adsorbents was evaluated by examining the breakthrough response. Figure
36.14 describes the breakthrough curves obtained for three inlet concentrations of As(III)
and As(V) in the column packed with PBFe-Act beads. The breakthrough time may be
deined as the time at which the concentration of the efluent stream increases to 1% of
the inlet concentration. For clarity, the enlarged section of the igure corresponding to the
breakthrough times for three concentrations (1, 2, and 3 ppm of arsenic) is now produced as
(a)
1.0
As(V)
concentration
(mg/l)
0.8
1
0.6
0.10
0.08
0.06
0.04
0.02
0.00
0 00 200
Time (min)
0.4
0.2
300
0.0
0
400
800
1200
1600
Time (min)
(b)
1.0
As(III)
concentration
(mg/l)
0.8
1
0.6
0.10
0.08
0.06
0.04
0.02
0.00
020406080 100
0.4
0.2
Time (min)
0.0
0
400
800
1200
1600
Time (min)
FIGURE 36.14
Breakthrough data for varying inlet arsenic concentration (amount of PBFe-Act = 1 g,
Q
= 0.003 l/min, pH 7 for
As(III) (b) and pH 6.5 for As(V) (a),
T
= 303 K). (From A.K. Sharma et al.,
Chem. Eng. Sci
., 65, 3591, 2010.)