Environmental Engineering Reference
In-Depth Information
(a)
200 nm*
(b)
(c)
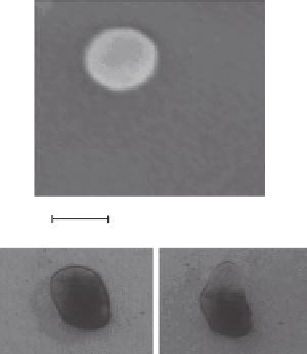
FIGURE 2.12
(a) FE-SEM of a cryofractured macroporous hydrogel showing a PANI nanosphere adsorbed on the surface
of the pore. (b) Photograph of a macroporous PNIPAM hydrogel functionalized with PANI nanoibers, before
thermal deswelling. (c) Photograph of a macroporous PNIPAM hydrogel functionalized with PANI nanoibers,
after thermal deswelling.
2.2.3.3 Polyaniline Nanoparticles Adsorbed Inside a Macroporous Hydrogel
Conducting polymer nanoparticles could be used to remove pollutants through a speciic
mechanism of metal ion reduction [126] or nucleophilic addition of the pollutant (e.g., thiol)
to the polymer [127]. However, free conducting polymer nanoparticles could end up in
natural waters and have toxic effects on aquatic life. Indeed, we have shown that PANI
nanoibers have teratogenic effects on frog larvae [128]. Therefore, a mechanism to use
conducting polymer nanoparticles without releasing them to the environment is desirable.
We have loaded PANI nanospheres and nanoibers into macroporous hydrogels [122]. The
nanoparticles are irreversibly adsorbed on the macropores (Figure 2.12a) and maintained
even under swelling/deswelling processes (Figure 2.12b,c). Therefore, the nanocomposite
could be used to remove toxic substances from water.
Since PANI nanoparticles are ~200 nm in size, preformed nanoparticles do not enter a
non-macroporous hydrogel owing to steric constraints.
2.3 Which Techniques Can Be Used to Measure the Relevant Properties?
Different techniques, including novel ones, have to be used to measure the properties of
nanoporous materials relevant to aquatic nanotechnology applications.
2.3.1 Surface Area and Porosity of Porous Carbon
The nanoporosity of porous carbon can be measured indirectly by gas (e.g., N
2
) adsorp-
tion isotherms. Figure 2.13 shows the experimental isotherm of a porous carbon and the