Environmental Engineering Reference
In-Depth Information
the water can also vary depending on the amount of time for a given process to complete
its cycle. Produced water can be in excess of 80°C immediately upon wellhead extraction.
However, temperature may drop to near freezing if water is stored above ground in cold
climates before treatment. The pH of produced water is less variable and often buffered by
dissolved salts (e.g.,
CO
2−
and
SO
2−
). Extraction of organic acids can be highly dependent
on pH, and thus understanding of how pH may affect treatment methods is critical to
achieving optimal performance.
Assessment of sensitivity of water treatment with respect to process variables was con-
ducted with Osorb across the range of values that may be anticipated with produced water.
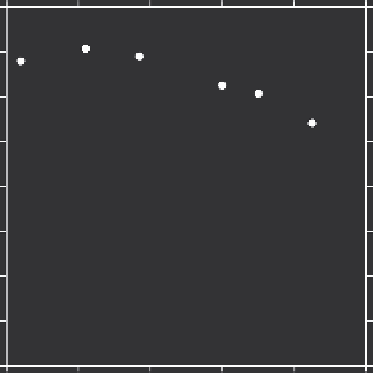
Temperature of an organic liquid has no impact on the degree Osorb swells; however, the
equilibrium partitioning from a solute dissolved in water may be dependent on tempera-
ture. The amount of extraction of 200 ppm toluene by 0.2% w/v
Osorb was measured at
various temperatures through batch equilibrium experiments employing gas chromatog-
raphy for analysis. The partition coeficient remained relatively high across all tempera-
ture ranges, declining slightly at temperatures above 60°C and below 10°C (Figure 8.6).
The same trend was also observed for the partitioning of 400 ppm 1-butanol from water,
indicating the phenomena is general and not dependent on polarity of the organic solute.
Whereas water temperature has little effect on extraction performance, increases in
salt concentration (TDS) greatly improve partitioning of dissolved solutes. For instance,
the partition coeficient for dissolved toluene (125 ppm) improves 10-fold when the TDS
increases from 0 ppm to 100,000 ppm (Table 8.2). At high TDS, it is possible to extract >99%
of dissolved toluene with 0.5% w/v Osorb, even at elevated temperatures. High TDS also
improves the extraction of methanol from water. Elevated ionic strength does not alter
the properties of the generally hydrophobic material, and the reason for the increase in
extraction eficiency is more likely the well-known “salting out” effect. In short, the addi-
tion of ions tends to order the water molecules, making them participate less in solvating
the dissolved organic. As a result, the organic is driven out of the aqueous phase either by
4.0
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
0
20
40
60
80
100
Te mperature (ºC)
FIGURE 8.6
Dissolved toluene partition coeficient as a function of water temperature. TDS = 0 ppm; toluene concentration =
200 ppm; 0.2% w/v Osorb.