Environmental Engineering Reference
In-Depth Information
Hydrophobic
barrier
(a)
(b)
Matrix tension
Void volume
New surface area
Dissolved hydrocarbons
(c)
(d)
Continued matrix expansion
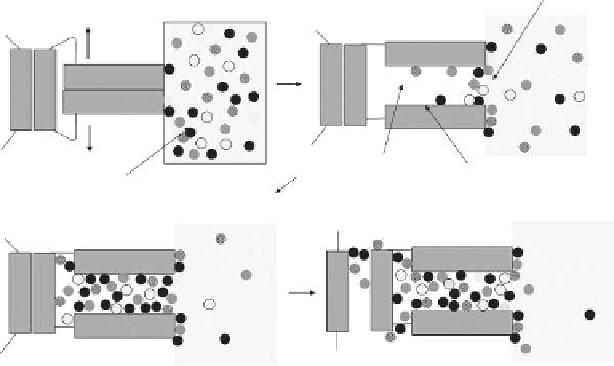
FIGURE 8.5
Proposed model for absorption of dissolved organics by swellable glass media. (a) Initial adsorption to the
surface of the material. (b) Suficient adsorption occurs to trigger matrix expansion leading to absorption
across the sorbent-water boundary. (c) Pore illing leading to further percolation into the nanoporous matrix.
(d) Continued matrix expansion increases available void volume.
matrix, leading to nonselective capture of organics beyond what could only be attributed
to physisorption. It was observed in previous work that the force produced during swell-
ing was not continuous with respect to swollen volume but exhibited a stepwise behavior.
16
Thus, it appears that once a particular amount of contaminant is
ad
sorbed it “unlatches”
the matrix to yield void volume for subsequent
ab
sorption events (Figure 8.5). In all cases,
log
k
> log
K
ow
; in other words, the partitioning into Osorb is greater than what would be
predicted on the basis of standard liquid-liquid extraction, suggesting that there is an addi-
tional thermodynamic driving force for absorption. A likely explanation for the enhanced
partition coeficients is that an increased Osorb entropy upon swelling can compensate for
entropic and enthalpic barriers to absorption. What is quite unique about Osorb is that sol-
utes condense as liquids (or solids depending on identity) in the Osorb matrix. Absorption
by Osorb is fundamentally condensation in the pore structure, not adsorption through
intermolecular forces of attraction between organic and the Osorb surface, although initial
absorption events serve to unlock the matrix. Evidence for condensation of absorbates has
been obtained using infrared spectroscopy to probe the physical state of captured solutes
within the matrix.
8.2.3 Effect of Process Conditions
Variations in salt concentration, temperature, and pH are typically encountered when
treating produced water streams. For instance, the total dissolved solids (TDS) in pro-
duced water can generally range from 5000 to 100,000 ppm, and in some cases can be
much higher. Sodium chloride is the most abundant dissolved solid, although group I and
group II metal sulfates are also relatively common. Treatment technologies need to be able
to handle high variability in the amount and types of dissolved solids. Temperature of