Environmental Engineering Reference
In-Depth Information
TABLE 8.1
Extraction of Various Co
mpounds from Water by Osorb
Degree of Extraction
Representative Compounds
>90%
Aliphatic hydrocarbons, toluene, benzene, carbon tetrachloride, perchloroethylene,
trichloroethylene, naphthalene
50%-90%
Methyl
t
-butyl ether, nonionic surfactants
coeficients for the absorption of organic species from water by Osorb range from 2.8 × 10
5
to 1.0 × 10
2
. A number of aspects distinguish Osorb from traditional solid adsorbents:
(i) equal to slightly higher absorbance capacity compared with activated carbon; (ii) the abil-
ity to absorb polar species such as MTBE, acetone, phenol, and 1-butanol from water; and
(iii) easy absorbent bed regeneration. The magnitude of the observed partition coeficients
for Osorb (
k
= [
X
] O s o r b/[
X
]water) follows absorbate polarity as assessed by
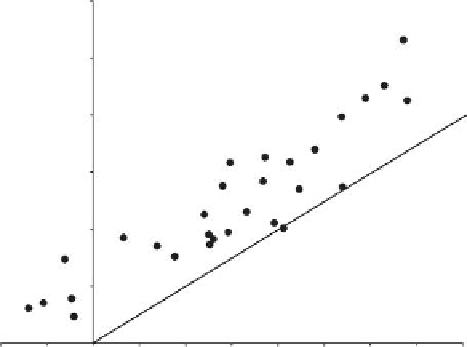
K
ow
(Figure
8.4), except for the most polar species. Another exception to this trend is toluene, which
has a slightly higher partition coeficient than predicted by a direct relationship upon
K
ow
.
Toluene exhibited the strongest binding afinity (
k
= 2.85 × 10
5
) when the concentration of
toluene was 25 ppm. At high toluene concentrations (530 ppm), Osorb absorbed 190 mg
of toluene per milligram of material. On the basis of surface area measurements of the
dry material using BET nitrogen absorption, it is estimated that the packed monolayer is
achieved at 150 mg/mg (toluene/Osorb)
16
; thus, expansion of the matrix must play a role
in the absorption under conditions of high loading. The partition coeficients for absorp-
tion of water-miscible organic solvents such as acetone and 1,4-dioxane do not follow a
relationship as predicted by
K
ow
and exhibit a higher degree of absorption than expected
based on polarity. Matrix expansion may also aid the binding of these polar species even
though they are fully soluble in water.
A substantial amount of the data (absorption isotherms, equilibrium binding, and break-
through curves) suggest a dynamic absorption behavior is derived from animation of the
6.0
Naphthalene
5.0
4.0
To luene
Benzene
3.0
2.0
Phenol
1.0
0.0
0.0
-1.0
-0.5
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
log
K
ow
FIGURE 8.4
Plot of the partition coeficient (log
k
) of a compound absorbed in Osorb vs. dissolved in water vs. the com-
pound's corresponding octanol-water partition coeficient (log
K
ow
).