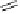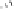Biology Reference
In-Depth Information
(
35
) is thought to act as an antioxidant protecting some volatile thiols
that contribute to the fruity aroma of these wines. Glutathione (
35
) is
known to engage in nucleophilic addition reactions with orthoquinones
derived from the oxidation of caffeoylated tartaric acids in wine musts
(Cheynier
et al.
, 1986, Cheynier
et al.
, 1990). This process, which is
implicated in the oxidative browning of musts, can certainly contribute
to the variations of concentrations of
35
in wine musts (
ca
. from 24 to
3 mg/L), but the apparent influence of aging in oak barrels on the
disappearance of
35
from the wine solution led us to contemplate its
participation in nucleophilic substitution reactions with oak
C
-glycosidic
ellagitannins.
+H
+
/-H
2
O
1
[
27
]
O
NH
2
N
HO
2
C
H
CO
2
H
-H
+
O
HS
35
: glutathione
HO
HO
2
C
HO
OH
HO
HN
O
O
O
HO
O
O
NH
HO
S
O
O
1
O
O
O
OH
HO
NH
2
O
O
HO
HO
2
C
OH
HO
OH
HO
OH
36
:
β
-1-
S
-glutathionyl vescalagin (51%)
Fig. 9.13
Hemisynthesis of β-1-
S
-glutathionyl vescalagin (
36
) from vescalagin (
1
) and
glutathione (
35
) in an acidic organic medium (isolated yield, see text).
HO
On the basis of previous observations made on the condensation of
vescalagin (
1
) with thiols (Jourdes, 2003), we surmised that the
glutathione (
35
) could react in a similar fashion to furnish β-1-
S
-
glutathionyl vescalagin (
36
). This was indeed verified under reaction
conditions similar to those used for the hemisynthesis of the acutissimins
(see Section 9.2.2) and the expected product
36
was obtained in an