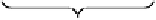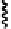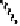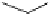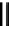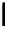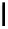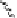Biology Reference
In-Depth Information
OH
OH
dehydrodigalloyl
ether core
G
'
OR
OH
=
O
G
'
O
OR
OH
G
'
O
O
G
'
O
O
OH
O
GO
O
GO
OH
O
RO
OG
'
OG
'
O
O
(
S
)-HHDP
GO
RO
GO
OR
OR
O
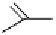
R = H, coriariin A (
62
)
R = CH
3
, nonacosa-
O
-methyl-coriariin (
63
)
OR
G =
OR
Fig. 6.12 Structure of coriariin A (
62
) and its methylated derivative
63
.
6.2.1.3
Other ellagitannins
Besides agrimoniin and coriariin A, the work of Miyamoto and
colleagues showed that several other tannins that met some particular
structural requirements were active against sarcoma-180 tumor cells
(Miyamoto
et al.
, 1987b, 1993a/b). From their structural-activity
relationship studies, some general trends emerged. Condensed tannins,
caffeic acid derivatives, bergenin derivatives, dehydroellagitannins, and
gallotannins exhibited little-to-no anticancer activity. In general,
monomeric ellagitannins were found to be less potent than dimeric ones.
Exceptions to this generalization are the two particularly active
monomers tellimagrandin II (
65
) with 3 out of 6 tumor regressors,
although with only a 18 %ILS at a 10 mg/kg dose, and rugosin A (
68
)
with one cured mouse out of six and a 110 %ILS at a 5 mg/kg dose (Fig.
6.13). The gallotannin penta-
O
-galloyl-β-
D
-glucose (β-PGG,
66
) was
relatively inactive with no regressors and an 82 %ILS at 10 mg/kg dose.
Compounds containing an open-chain glucose unit also were ineffective.
Several macrocyclic ellagitannins such as the trimer oenothein A (
69
) (1
regressor/6 mice and 103 %ILS at 10 mg/kg) and the dimer oenothein B
(
67
) (4 regressors/6 mice and 196 %ILS at 10 mg/kg) were very active.
Exposed phenolic hydroxyls were necessary for activity, as nocacosa-
O
-