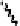Biology Reference
In-Depth Information
hydrocarbon receptor (a nuclear transcription factor) inhibition (EC
70
6.4
μM), anti KO
2
-induced histamine release (IC
50
0.68 μM), and tyrosinase
(target for insect control) inhibition (ID
50
61 μM) (Hrenn
et al.
, 2006,
Kolodziej
et al.
, 2001, Amakura
et al.
, 2003, Kanoh
et al.
, 2000, Kubo
et al.
, 1995, Ito
et al.
, 2001).
OH
OH
OH
HO
G
'
OH
=
G
'
O
O
G
'
O
O
O
OH
G
'
O
O
O
OG
'
OG
'
G'O
OH
O
O
G'O
OH
O
G'O
G'O
OH
OH
agrimoniin (
61
)
OH
Fig. 6.11 Structure of agrimoniin (
61
).
6.2.1.2
Coriariin A
Among the 63 tannins initially surveyed by Okuda's and Miyamoto's
groups, coriariin A (
62
, Fig. 6.12) showed the strongest activity against
sarcoma-180 tumor cells with a 238 %ILS and tumor regression in 3 out
6 mice at a 5 mg/kg dose (Miyamoto
et al.
, 1987b). Coriariin A, a dimer
of tellimagrandin II (
65
), was isolated from the Japanese poisonous plant
Coriaria japonica
A. Gray (Hatano
et al.
, 1986). Coriariin A, along with
several oligomeric ellagitannins, was found to be a potent inhibitor of
both poly(ADP-ribose) glycohydrolase (IC
50
8.5 μM) and of histamine
release (IC
50
2.97 μM) (Aoki
et al.
, 1993, Maruta
et al.
, 2007, Kanoh
et
al.
, 2000). Coriariin A also has anti-herpes simplex virus activity (ED
50
0.038 μg/mL) (Fukuchi
et al.
, 1989). The chemical synthesis of coriariin
A has been accomplished (Feldman
et al.
, 2000, Feldman and Lawlor,
2000, see Section 5.2.2.6 in Chapter 5). Further examination of other
related tannins (now totaling 108) at different doses and through
different methods of administration has led to the identification of
several other tumoricidal compounds. The best results were found when
the tannins were administered to mice through intraperitoneal injection 4
days before treatment with cancer cells (Miyamoto
et al.
, 1987a).