Environmental Engineering Reference
In-Depth Information
Table 11.2 List of common elements that can be analyzed by stripping voltammetry
By anodic stripping voltammetry:
Ag
a
, As, Au
a
, Ba, Bi, Cd, Cu, Ga, Ge, Hg
a
, In, K, Mn, Ni, Pb, Pt, Sb, Sn, Tl, Zn
By cathodic stripping voltammetry:
b
Br
,Cl
,I
,S
2
, thio compounds
a
Determined on solid electrodes, such as carbon or gold.
b
These form mercury precipitates on the electrode, which are subsequently stripped off in a negative scan
[Rubinson and Rubinson (2000)].
Let us illustrate the analytical principles by assuming that Cd and Cu are the two
elements of interest in the solution. The first step in ASV is to preconcentrate both
metals from the solution into or onto a microelectrode (with a large surface area) by
an electrodeposition process. The remarkably low detection limit of stripping
voltammetry is attributable to the preconcentration step. The second step, called
stripping,istostrip the metals by applying changing potential (Fig. 11.7a). The
current vs. potential plot (I vs. E) during the stripping process (Fig. 11.7b) is
obtained for quantitative analysis.
In anodic stripping voltammetry, the microelectrode behaves as a cathode where
metal ions Cd
2þ
and Cu
2þ
are reduced into metal in their metallic forms (Cd and Cu).
Deposition
-1.0
M
2+
+
2e
-
M
-0.8
-0.6
-0.4
(a)
-0.2
0.0
Time
Cd
2+
+
2e
-
Cd
Cu
2+
+
2e
-
Cu
(b)
0.0
-1.0
-0.8
-0.6
-0.4
-0.2
Potential (V)
Figure 11.7
Anodic stripping voltammetry (ASV) in a solution containing Cd
2þ
and Cu
2þ
.
(a) Potential applied as a function of time. (b) Current vs. potential plot (voltammogram) showing two
peaks containing two metals of interest (Cd and Cu). From Principles of Instrumental Analysis 5th
edition by Skoog et. al., 1998. Reprinted with permission of Books/Cole. A division of Thomson
Learning: www.thomsonrights.com.

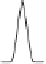


















Search WWH ::

Custom Search