Environmental Engineering Reference
In-Depth Information
(a)
(b)
End
point
End point
Volume of titrant
Volume of titrant
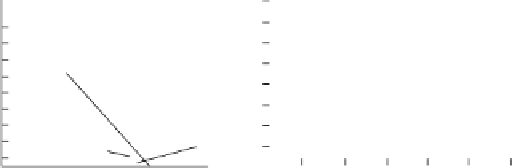
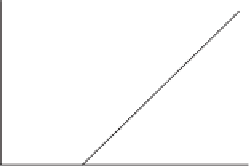
Figure 11.6
Measurement of chlorine by amperometric titration: (a) Forward amperometric titration,
(b) Back amperometric titration (Courtesy of Hach Company)
accurately deliver the titrant solution. The output of the amperometric titration,
unlike potential titration, is a plot of current vs. titrant volume (Fig. 11.6). As shown
in the figure, the abrupt change in current is used as the end point. The concentration
of chlorine present in the sample can be calculated by determining the exact amount
of titrant (PhAsO) added to extinguish the probe, current.
The titration described above is referred to as forward titration (Fig. 11.6a). For
water samples containing potential interference, a back titration is preferred, in
which a known excess amount of the reducing agent (Na
2
S
2
O
3
or PhAsO) is added to
the sample. After the reducing agent has reacted with the free and residual chorine in
the sample, the amount of remaining titrant is determined through titration with the
standard iodine (I
2
) solution. The total chlorine is then calculated on the basis of
Na
2
S
2
O
3
or PhAsO remaining. The back amperometric end point is signaled when
iodide (I
) is present, which is indicated by a current flow between the electrodes
(Fig. 11.6b). The titration reaction is
I
2
þ2S
2
O
2
3
ðexcessÞ
!
2I
þS
4
O
2
6
ð11
:
16Þ
By adjusting the reaction condition (pH), the above amperometric titration can be
used to further differentiate free chlorine and various residual chlorine compounds
(chloramines). The amperometric titration requires more skills and care than the
volumetric titration using color indicators.
11.3.3 Measurement of Metals by Anodic Stripping
Voltammetry (ASV)
Stripping voltammetry is an elegant electroanalytical technique with the promise of
extremely low detection limits for the analyses of both anions and cations. Cations
are stripped from anodes and anions are stripped from cathodes, hence the name
anodic stripping voltammetry and cathodic stripping voltammetry. Table 11.2 is a
list of common elements that can be determined by anodic and cathodic stripping
voltammetry. In environmental analysis, the former is of particular importance
because of its ability to analyze multiple metals in a single run. Anodic stripping
voltammetry (ASV), therefore, will be the focus of the following discussions.













Search WWH ::

Custom Search