Environmental Engineering Reference
In-Depth Information
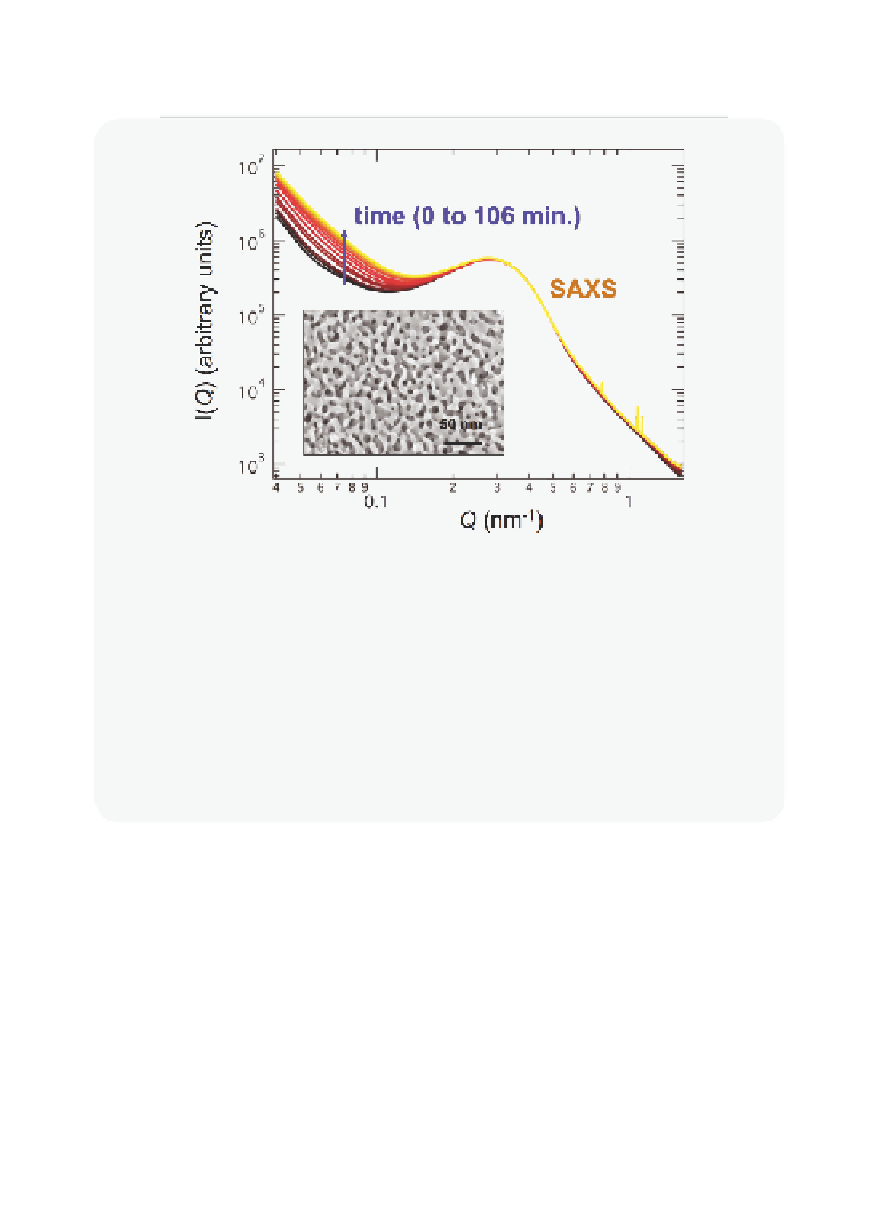
Figure 10.2.13
Inhibition of CaCO
3
precipitation in nanoporous silica
Experimental data suggest that CaCO
3
precipitation may be thermodynamically inhibited
in silica nanopores. The inset fi gure is an SEM image of the controlled pore glass CPG-75
showing the 7.5 nm diameter nanopores in this medium. The main fi gure shows the evo-
lution of the small angle X-ray scattering (SAXS) spectrum of the porous medium upon
exposure to a solution supersaturated with respect to calcite at 90
°
C. The peak at 3 nm
−
1
in the SAXS spectrum (caused by scattering by the nanopores) is unchanged during the
experiment, indicating that CaCO
3
does not precipitate in the nanopores. The change in
the SAXS spectrum at small
Q
values shows that CaCO
3
precipitates on the outside of
the CPG-75 grains.
Images courtesy of Andrew Stack, Oak Ridge National Laboratory.
Reactions in adsorbed water fi lms
Finally, fi eld-scale models assume that the CO
2
-rich phase does not
interact directly with solid surfaces: i.e., that CO
2
-mineral interactions are
always mediated by the aqueous phase. This assumption is consistent
with the expectation that most solid surfaces in carbon sequestration
sites are hydrophilic, hence at low capillary pressures they should be
coated with a fi lm of adsorbed or capillary water. However, in certain
regions of a CO
2
storage site (near the injection well, where dry CO
2
is
continuously injected, and near the top of a thick CO
2
plume, where cap-
illary pressure is high) these water fi lms may be extremely thin. Indeed,
studies of mineral weathering reactions suggest that the aquatic











Search WWH ::

Custom Search