Environmental Engineering Reference
In-Depth Information
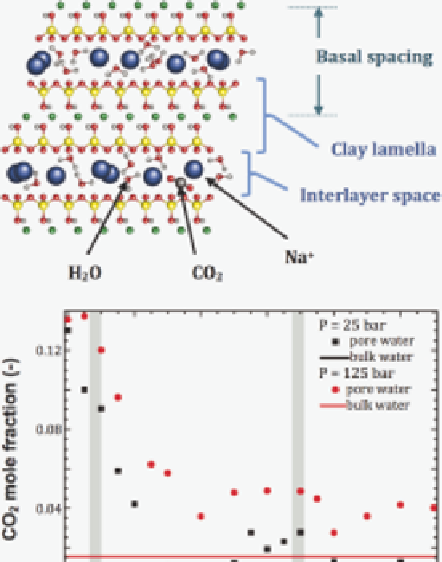
Figure 10.2.12
CO
2
solubility in smectite clay interlayer nanopores
Molecular simulations suggest that CO
2
solubility is higher in smectite interlayer nanopo-
res than in bulk water. The upper part of the fi gure is an illustration of the structure of
hydrated smectite clay. The lower part of the fi gure shows predictions of the solubility of
CO
2
in the nanopore water (squares) and in bulk liquid water (horizontal lines) as a function
of basal spacing (the width of one lamella plus one interlayer nanopore) at
P
=
2.5 MPa
(black) and 12.5 MPa (red). The vertical shaded bars indicate stable swelling states where
the nanopores contain one or two statistical water monolayers.
Figures reproduced with
permission from Botan et al.
[10.25].
Copyright
(2010),
American Chemical Society
.
One implication for GCS is that if solid carbonates precipitate in a clay-
shale caprock, as predicted under certain conditions [10.27], this precipi-
tation may preferentially clog the largest pores in the rock and enhance
its sealing properties.












Search WWH ::

Custom Search