Environmental Engineering Reference
In-Depth Information
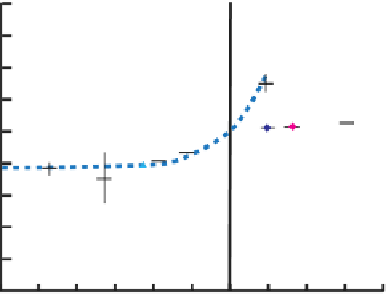
crystalline phases. The nucleation of solid phases or growth sites, rather
than the binding of solutes at existing growth sites, is increasingly recog-
nized as a key factor that controls mineral precipitation rates. This is illus-
trated in
Figure 9.8.6
, which shows that kaolinite dissolution at pH 4 and
22
C is well described by the rate equations, but kaolinite growth at the
same conditions is much better described by a model that accounts for
the rate of nucleation of growth sites on the kaolinite surface [9.39].
°
Dissolution at
in-situ
conditions
The long-term weathering rates of minerals at geological conditions can
be reconstructed from a range of sources. For example, silicate glass
weathering experiments with durations of tens of years have been carried
4 x 10
-13
Precipation
3 x 10
-13
Short term
2 x 10
-13
Long term
1 x 10
-13
0 x 10
-13
-1 x 10
-13
-2 x 10
-13
-3 x 10
-13
-4 x 10
-13
Equilibrium
Dissolution
-5 x 10
-13
-30
-25
-20
-15
-10
-5
0
5
10
15
20
∆
G (kJ/mol)
Figure 9.8.6
Dissolution and precipitation rates of kaolinite
Dissolution and precipitation rates of kaolinite as a function of the Gibbs free energy of reac-
tion at pH 4 and 22
C. The symbols represent measured values of the steady-state reaction
rate. The dashed blue curve was obtained by fi tting the rate equation described in this sec-
tion (with
n
°
1) to the dissolution data. The dashed red curve was obtained by
fi tting a two-dimensional nucleation model to the precipitation data. At
=
0.5 and
m
=
5 kJ/mol two
experimental values are reported on the graph: the faster growth rate was observed on a
time scale of hours for a kaolinite that had previously been subjected to steady-state dis-
solution for fi ve months and the slower growth rate was observed on longer time scales.
Figure redrawn from Yang and Steefel
[9.39]
.
∆
G
r
≈

















Search WWH ::

Custom Search