Environmental Engineering Reference
In-Depth Information
phyllosilicate dissolution rates normalized to the N
2
-BET surface area of
dry powders exhibit a large scatter, suggesting that this surface area is
a poor measure of reactive surface area. Dissolution rates show much
less scatter if they are normalized to the edge surface area of the clay
particles, i.e., the highly-reactive surface exposed at the edges of the
clay fl akes, measured by atomic force microscopy or by high-resolution
low-pressure gas adsorption. This is consistent with direct observations
of the dissolution of individual smectite clay particles, where the reaction
occurs primarily on the edge surfaces (
Figure 9.8.5
) [9.38].
A second key concept is that minerals do not grow only by a simple
process of solute attachment and detachment at growth sites; in reality,
their growth is much more complex. For example, mineral precipitation
may involve the formation of metastable precursors such as amorphous
silica or amorphous calcium carbonates that later slowly transform into
(d)
a
b
a
particle in (a)
(0h0m0s)
particle in (c)
(2h39m55s)
a
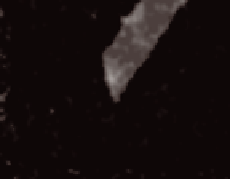
Figure 9.8.5
Dissolution of a single smectite lamellum
Superposition of two AFM (atomic force microscopy) images of a single smectite lamel-
lum observed before and after 160 minutes of dissolution in a 0.01 M NaOH solution. The
basal plane of the particle lies within the plane of the image; the thickness of the particle
in the direction normal to the plane of the image is 1 nm. The fi gure shows that dissolu-
tion occurs on the edge surfaces and different edge faces have different dissolution
rates.
Figure reproduced from Kuwahara
[9.38],
with permission from the Mineralogical
Society of America.

















Search WWH ::

Custom Search