Environmental Engineering Reference
In-Depth Information
gas phase
interface
liquid phase
CO
2
CO
2
CO
2
+B
BCO
2
Step 1:
diffusion
Step 2:
dissolution
Step 3:
diffusion
Step 4:
chemical reaction
Figure 5.4.4
Mass transfer from the gas to liquid phase
As we think about the reaction rate limitation, we can use a similar
approach to the one we used for diffusion limitation. If we construct
another mass balance for our control volume, we get:
()
2
()
dc
z
d c
z
CO
CO
2
2
=
D
CO
dt
2
2
dz
Due to the fact that we now have a chemical reaction, CO
2
can disappear
in our volume, according to the rate equation:
()
dc
z
CO
2
=
kc c
rB
CO
dt
2
In this rate equation,
k
r
is the reaction rate of CO
2
with B, which we
assume to be irreversible. Substitution in our mass balance equation
gives us:
()
2
dc
z
kc
CO
rB
2
=
c
=
k c
′
,
CO
CO
2
D
2
2
dz
CO
2
c
CO
2
and c
CO
2
(L)
c
CO
2
(see
with boundary conditions c
CO
2
(0)
=
=
Figure 5.4.2
). This equation has as solution:
(
)
(
)
()
c
z
=
A
exp
k z
′
+
B
exp
−
k z
′
CO
2





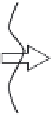






Search WWH ::

Custom Search