Environmental Engineering Reference
In-Depth Information
Question 5.4.1 Diffusion or reaction
In the diffusion-limited equation we see the reaction rate, in the reaction-limited
equation we see the diffusion coeffi cient. Does this make sense?
From the boundary conditions we have:
(
)
(
)
∗
0
∗
0
c
−
c
exp
−
k L
′
c
−
c
exp
−
k L
′
CO
CO
CO
CO
2
2
A
=
2
=
2
(
)
(
)
(
)
exp
kL
′
−
exp
−
kL
′
2 sin
h
−
kL
′
(
)
(
)
∗
0
∗
0
c
−
c
exp
k L
′
−
c
+
c
exp
k L
′
CO
CO
CO
CO
2
2
2
2
B
=
=
(
)
(
)
(
)
exp
kL
′
−
exp
−
kL
′
2 sin
h
kL
′
The fl ux is given by:
(
)
(
)
()
j
z
=−
DAk
′
exp
k z
′
−
Bk
′
exp
−
k z
′
CO
CO
2
2
Of particular interest is the fl ux at
z
=
0:
()
[
]
j
0
=−
D
k
′
A
−
B
CO
CO
2
2
Dk
′
(
)
(
)
CO
0
∗
2
c
cos
h
k L
c
=
′
−
(
)
CO
CO
2
sin
hkL
′
2
This equation looks complex, but we can learn a lot by looking at two
limiting cases.
Let us fi rst assume that the reaction is slow compared to diffusion:
kc
rB
kL
′
=
L
1
D
CO
2


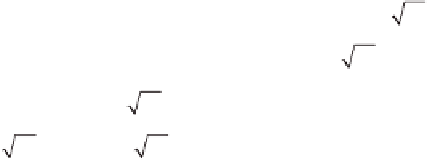
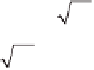











Search WWH ::

Custom Search