Environmental Engineering Reference
In-Depth Information
from the system. Contrary to this, in a recirculation system, the participation of the in-situ
produced H
2
SO
4
can quite important. The involvement of the in-situ produced HNO
3
was reported by Marafi et al.
[480]
in the study using the mixture of oxalic acid with
Al(NO
3
)
3
.
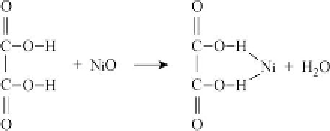
Besides reacting with anions in solution, the metal dissolution may proceed via liquid-solid
reaction, which may involve a direct interaction of the non-ionized acid with metal oxide on
the surface of catalyst, yielding a complex 2 shown in Reaction
{7.2}
. This reaction is
always accompanied by the formation of H
2
O. Such a dissolution may also involve two
non-ionized molecules of acid. To depict these events, again oxalic acid and NiO are used as
an example.
{
7.6
}
Compared with the other acids (
Fig. 7.9
), oxalic acid would be the least sterically hindered
during the metal oxide dissolution. Moreover, an intramolecular rearrangement such as
required during the formation of complex 2 from complex 1 in Reaction
{7.2}
is more easily
attainable compared with more complex acids. Therefore, besides the strength of acid, the
structure of organic acids may be an important factor while selecting the most efficient
leaching agents. Of course, Reaction
{7.6}
may not proceed without the ionization of the acid
in true sense, although this process is not depicted. Most likely, the final complex in Reaction
{7.6}
arose from a transition state in which the NiO aided weakening of O H bonds resulted
in the H
+
release while Ni
2+
partially coordinated with the oxygen in OH groups. As the last
step, H
+
coordinated with the O in NiO to form H
2
O while the final complex in Reaction
{7.6}
was liberated from the transition state.
Reactions
{7.1}
to
{7.3}
assumed the presence of metallic species in a dissolved form in
solution, whereas Reaction
{7.6}
in a solid oxidic form. This may approach the conditions for
either decoked catalyst or a sulfided catalyst leached in the presence of an oxidizing agent.
Leaching of a coked catalyst in the absence of an oxidizing agent may involve a similar set of
reactions as proposed for the oxidic form of metals with MeS
X
replacing MeO
X
species
[483]
.
Because of a low solubility of the former, the dissolution near or on the surface such as shown
in Reaction
{7.6}
for the oxidic catalyst is expected to be much more pronounced and may
proceed according to the following general equations:
4H
+
+
4(HOOC-COO
−
)
Me(HOOC-COO
−
)
4
+
4(COOH)
2
†
+
MeS
2
→
H
2
S
{7.7}
4H
+
+
2(
−
OOC-COO
−
)
Me(
−
OOC-COO
−
)
2
+
2(COOH)
2
†
+
MeS
2
→
2H
2
S
{7.8}