Environmental Engineering Reference
In-Depth Information
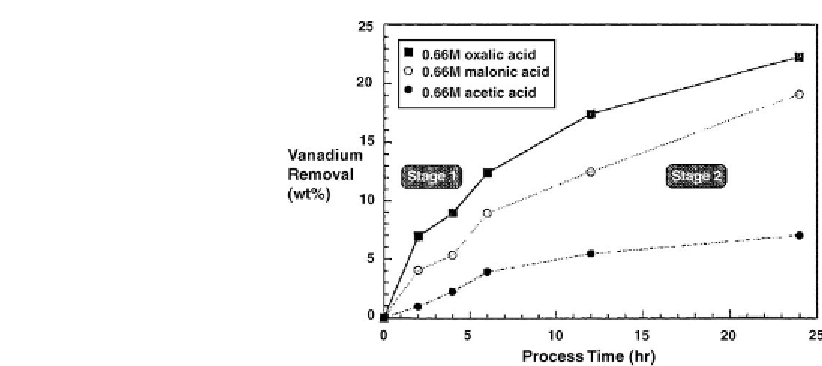
Figure 7.10: Effect of type of organic acid (0.66M) on vanadium removal at 50
C.
: oxalic acid;
: malonic acid;
: acetic acid [From ref.
490
. Reprinted with permission].
complex formation in solution will increase in the following order:
Acetic
∼
butyric
<
glycolic
∼
lactic
<
tartaric
∼
citric
<
malonic
oxalic
The results on V removal in
Fig. 7.10 [490]
are in the agreement with these trends. The study
of Reda
[489]
confirmed similar trends as well. However, different trends were observed for Ni
removal
[480-482]
. For example, for Ni, malonic acid was much more efficient leaching agent
than oxalic acid. According to Reaction
{7.2}
, two carboxylic groups are required to form a
complex. Then, a complex can also be formed using two molecules of a partially ionized acid
as shown in Reaction
{7.3}
.
It has been generally observed that the solubility of metal oxides in water is significantly
greater than that of the corresponding metal sulfides. To a certain degree, beneficial effects of
the oxidizing agents on leachability may be attributed to the conversion of a sulfidic form of
metals to an oxidic form according to the following tentative reaction in which H
2
O
2
is used as
the model oxidant:
MeS
X
+
2H
2
O
2
⇒
MeS
X
−
1
O
+
SO
2
+
H
2
O
{7.4}
H
2
O
2
+
SO
2
⇒
SO
3
+
H
2
O
⇒
H
2
SO
4
{7.5}
The oxidation process may continue until MeS
X
is completely oxidized to MeO
X
. The release
of SO
2
in Reaction
{7.4}
should be noted. If sufficient concentration of H
2
O
2
is maintained,
SO
2
can be oxidized to SO
3
and subsequently converted to H
2
SO
4
via Reaction
{7.5}
. The
participation of the in-situ produced H
2
SO
4
during leaching cannot be ruled out. However,
when a flow reactor is employed, the H
2
SO
4
build-up is prevented by its continuous removal