Environmental Engineering Reference
In-Depth Information
ion-atom polarization scattering that is connected with atom capture by an ion, so
the difference between the mobilities for atomic and molecular ions is not large at
low temperatures. In contrast, at high temperatures or electric field strengths, the
mobility of an atomic ion is determined by the resonant charge exchange process,
and the cross section of this process is almost constant if the temperature or elec-
tric field strength increases, whereas the mobility of a molecular ion is determined
by the ion-molecule polarization interaction, and the cross section of this process
decreases with increasing temperature or electric field strength. Therefore, the dif-
ference in the mobilities of molecular and atomic ions increases with increasing
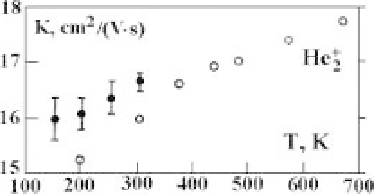
temperature or electric field strength. Figure 4.16 represents the temperature de-
pendence of the mobility of molecular helium ions in helium according to experi-
ments [59, 60]. Comparing this dependence with that for atomic helium ions given
in Figure 4.7, one can see that at a temperature of 100 K the mobilities of atomic
and molecular ions are similar, whereas at a temperature of 700 K the mobility of
molecular ions is about twice that of atomic ions.
One more aspect of transition between atomic and molecular ions in a plasma
of inert gases is connected with a different rate of electron-ion recombination for
atomic and molecular ions. Because molecular ions partake in dissociative recom-
bination, and recombination of atomic ions results from the three body process,
the rate of recombination of electrons and ions in these two cases differs dramati-
cally. Therefore, the rate of electron-ion recombination in a plasma of inert gases
may be determined by conversion of atomic ions in molecular ones.
Competition between processes in molecular gases involving different types of
ions is more complicated than that in a plasma of inert gases. In particular, if H
2
ions are formed as a result of ionization of hydrogen molecules in a hydrogen gas,
these molecular ions partake in the following ion-molecule reaction:
H
2
C
H
3
C
H
2
!
H
C
1.7 eV .
(4.108)
10
9
cm
3
/s and
The rate constant for this process at room temperature is 2.1
in the case of the process D
2
C
D
2
, the rate constant for D
3
formation is
10
9
cm
3
/s [73]. These rate constants are similar to the rate constants for
polarization capture of ions which follow from (2.40) for the cross section of ion-
molecule polarization capture and are similar to those obtained in other experi-
ments. From process (4.108) it follows that H
3
1.6
is the basic ion type in hydrogen at
Figure 4.16
The temperature dependence of the mobility of molecular helium ions in helium
according to experiments [59] (open circles) and [60] (filled circles).