Chemistry Reference
In-Depth Information
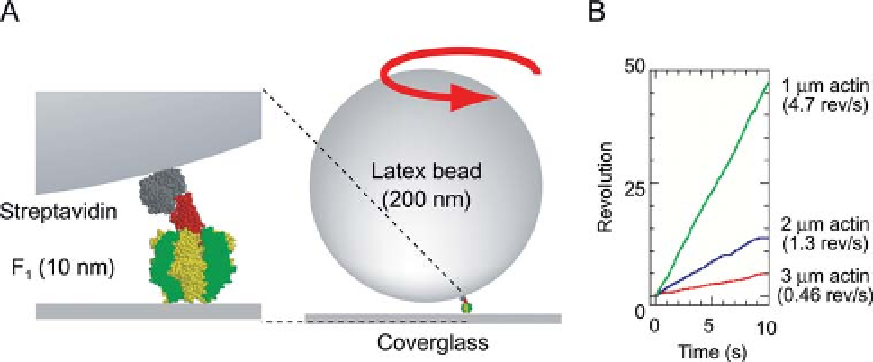
Figure 10.4 The experimental system for single-molecule
observation of the rotation of F
1
. (A) The
a
3
b
3
ring is fixed on the
glass surface to suppress the lateral and rotational Brownian
motion of the F
1
molecule. A large probe, for example, actin
filaments and latex beads, was attached to the g subunit to
visualize rotation. (B) Example of the dependence of the rotational
speed on the length of the actin filament at 2mM ATP.
from angular velocity and the drag coef
cient was constant and approximately
40 pNnm. If we assume that the torque is generated at the interface between the
b
subunits in a radius of 1 nm, it corresponds to a force of 40 pN. This value is
considerably larger than that generated by most of the known nucleotide-driven
molecular motors such as kinesin (
and
g
6 pN) [13], myosin (
4 pN) [14], and RNA
polymerase (
14 pN) [15]. The only known nucleotide-drivenmotor stronger than F
1
is the portal motor of bacteriophage (
57 pN) which packages DNA inside the virus
against a large internal pressure [16].
In addition to ATP, the rotation of F
1
was also supported by other purine
nucleotides (guanosine triphosphate and inosine triphosphate) that generated torque
comparable to that of ATP but not by pyrimidine nucleotides (cytidine triphosphate
and uridine triphosphate) [17]. This result suggests that the mechanical character-
istics of rotation are inherent in the F
1
structure, and purine nucleotides but not
pyrimidine nucleotides can trigger and maintain the rotation. Truncation of the
carboxyl-terminal 21-amino acid residues of the
subunit decreased the torque by
50% (20 pNnm), indicating that the torque is actually generated by mechanical
interaction between the
g
b
and
g
subunits [18]. The reconstitution of the
e
subunit to
the
a
3
b
3
g
subcomplex did not affect the torque [19].
10.2.1.3 Steps in Rotation
At low [ATP] in which ATP binding was rate-limiting, the rotation became stepwise
(Figure 10.5A) [12]. The step size was 120
, which was consistent with the pseudo-
threefold symmetric structure of F
1
. The 120
stepping rotation is an intrinsic feature
since the angle-resolved imaging of a single
uorophore attached to the
g
subunit also