Chemistry Reference
In-Depth Information
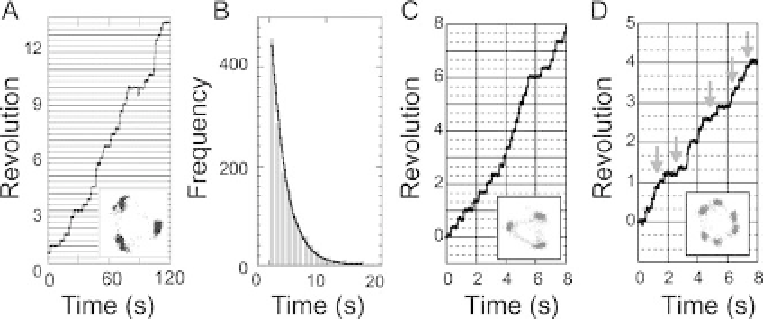
Figure 10.5 Steps in the rotation of F
1
. (A) Rotation of F
1
with 120
steps observed at 20 nM ATP. (B) Histogram of the dwell time
between 120
steps at 20 nM ATP. The histogram was fitted by a
single exponential function (solid line). (C) Rotation of a mutant
F
1
(E190D) with 120
steps at 2mM ATP. (D) Rotation of F
1
(E190D) with 80
and 40
substeps at 2
m
MATP. The dwells at the
80
substep are indicated by arrows.
revealed 120
steps [20]. At low [ATP], the rotational speed is proportional to
concentration down to 200 pM, suggesting that the same rotary mechanism is
functional even at very low [ATP] [21]. The histogram of the dwell time between
the steps was well
fitted by a single exponential function, indicating that each step is
driven by one ATP molecule (Figure 10.5B). This conclusion was further supported
by the comparison of the rotational speed with the turnover rate of ATPase at low
[ATP]; the value of the former was in good agreement with the one-third value of
the latter.
The large probe limited the maximum rotational speed of F
1
to 6
-
8 revolutions per
second (rps). When the large probe of rotation was replaced with a smaller one such
as a colloidal gold particle with a diameter of 40 nm, the viscous load became
negligible and faster rotation was observed [22]. At high [ATP], the rotational speed
reached 130 rps, which is comparable to that expected fromthemaximumrate of ATP
hydrolysis (
300 s
1
at 25
C). At a recording rate of 8000 frames/s, 120
steps were
observed even at 2mM ATP. These steps are not driven by ATP binding since the
binding rate (k
on
) of ATP is around 2
-
3
10
7
M
1
s
1
(this corresponds to a binding
dwell time of 17
-
25
s at 2mM) and they could not be detected at the above-
mentioned recording rate. When [ATP] was decreased, 120
steps were resolved
into
m
30
substeps. While the dwell time before the 90
substep was
inversely proportional to [ATP], no change was observed in the dwell time before the
30
substep. This implies that the 90
substep is driven by ATP binding, while the 30
substep is driven by other events. Statistical analysis of the dwell time before the
30
substep indicated that at least two events, each of 1ms duration, occur before
substepping.
When the rotation of an F
1
mutant with a very low maximum ATP hydrolysis rate
90
and
3s
1
) was driven by high [ATP], 120
steps were observed at the video rate of
(2