Environmental Engineering Reference
In-Depth Information
Share
1,0
Aerobic respiration
Denitrification
Manganese
r
eduction
Iron (hydr)oxide reduction
Sulfate reduction
Methanogenesis
0,9
0,8
0,7
0,6
0,5
0,4
0,3
0,2
0,1
0,0
0,0
0,5
1,0
1,5
2,0
2,5
3,0
3,5
4,0
Depth [cm]
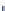
Fig. 9.5 Redox-sequence in aquatic sediments, see van Cappellen and Wang (
1995
,
1996
);
recalculated by the author
requires some knowledge of their consumption and reproduction behavior, which is
seldom available.
It is an important characteristic of redox reactions that most environments show
a distinctive preference for a special redox reaction, depending on the biogeochem-
ical conditions. The numbering in Table
9.1
provides the preference priority. As
long as oxygen is abundantly available in a system, all other redox reactions play
a minor role. If there is no or only a small amount of oxygen present, nitrate takes
the leading role. If there is also no nitrate, manganese and iron oxide reduction
become important and so forth.
The example in Fig.
9.5
results from measurement and modeling studies in
aquatic sediments, published by van Cappellen and Wang (
1995
,
1996
). The
relevant redox parameters change within the first centimeters below the sediment
water interface ('depth' in the figure). The figure depicts the share of specific redox
processes on the total reaction dynamics as a function of depth below the water
sediment interface. In the upper half centimeter, in the aerobic zone where oxygen
is present, aerobic respiration dominates over all other processes. Below follows
a zone where the share of reactions consuming oxygen becomes less important,
increasingly admitting all other redox interactions to take place. Denitrification is
the first competing process but is relevant only in a narrow zone of a few
millimeters length and always remaining with a share of less than 30%. Iron and
manganese reduction become relevant at the same depth, but, due to the higher
abundance of iron (hydr)oxides, the former redox process remains more relevant
within the rest of the depth interval. Sulphate reduction is the only process that
gains relevance with increasing depth. Obviously, in this set-up sulphur is available
at such high concentrations that methane formation is suppressed everywhere.
In other environmental compartments the extensions of redox zones may have
a different length scale than in the given example. Holzbecher et al. (
2001
) present