Biology Reference
In-Depth Information
100
EGTA
4
−
H
2
EGTA
2
−
50
HEGTA
3
−
0
6
7
8
9
pH
10
11
12
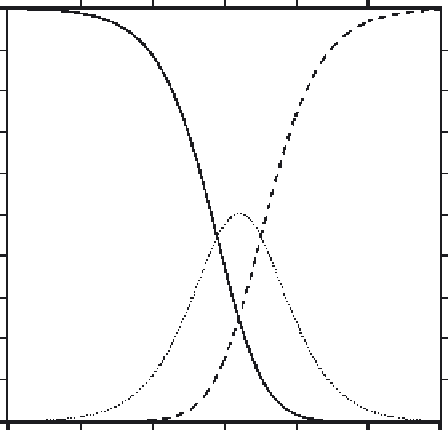
Fig. 7
Percentage of free EGTA existing as H
2
EGTA
2
, HEGTA
3
, and EGTA
4
as a function of
solution pH. Calculations performed with data for EGTA (at 0.1 M ionic strength, 25
C) tabulated by
Martell and Smith (1974)
; see
Appendix 1
for algebraic details.
knowing that the two highest pK
a
s of BAPTA are 5.47 and 6.36 (
Tsien, 1980
), one
infers that the ability of BAPTA to bind Ca
2
þ
should be only very weakly
dependent on pH, as shown in
Fig. 8
. Comparison of the two traces in
Fig. 8
shows that BAPTA has the advantage of being only weakly pH dependent in the
physiological pH range. The fact that the two traces cross between pH 7.2 and 7.3
implies that EGTA has the potential advantage of being a progressively stronger
binder of Ca
2
þ
above the crossover point (e.g., about two- and ninefold stronger
than BAPTA at pH 7.5 and 7.8, respectively). The pH insensitivity of BAPTA
makes it a less troublesome Ca
2
þ
bu
V
er to use, although it is more costly than
EGTA.
B. Lowering Extracellular [Ca
2
þ
]
In an experiment, lowering extracellular [Ca
2
þ
] is often desirable. Depending on
how low one wishes to clamp the extracellular [Ca
2
þ
], one of the approaches
described in the following sections may be adopted. The procedures require either
a stock solution of 1 M Na
2
H
2
EGTA at a pH near neutral or a stock solution of
1MNa
4
BAPTA.