Biology Reference
In-Depth Information
12
11
EGTA
10
9
8
7
BAPTA
6
5
4
6
7
8
9
pH
10
11
12
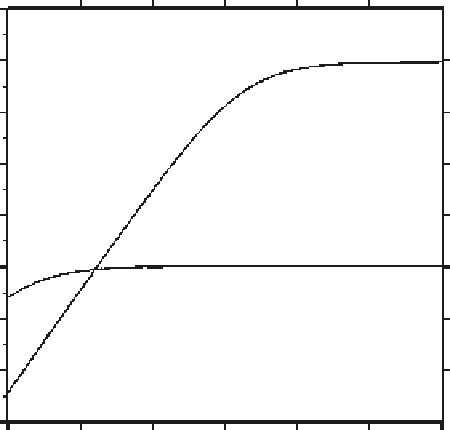
Fig. 8
Plot of pK
0
d(Ca) versus pH for EGTA and BAPTA. Calculations performed with data for
EGTA from
Martell and Smith (1974)
and for BAPTA from
Tsien (1980)
.
1. Lowering [Ca
2
þ
]to
1
m
M but not Approaching 0
a. Using EGTA
Na
2
H
2
EGTA can be added directly to calcium-containing medium at a concen-
tration that is 3-4 times
15
the concentration of Ca
2
þ
in the medium. Because binding
of Ca
2
þ
to H
2
EGTA
2
releases protons and acidifies the medium, simultaneously
adding TRIS base [tris(hydroxylmethyl)aminomethane] at a concentration equal to
<
2.2 times the Ca
2
þ
concentration in the medium is also necessary. This amount of
TRIS scavenges protons released from H
2
EGTA
2
and maintains the pH of the
medium at nearly the level before EGTA addition. This procedure can be applied
confidently down to pH
6.8. At pH levels that are much lower, the strong pH
sensitivity weakens EGTA and makes it inconvenient to use as a Ca
2
þ
scavenger.
b. Using BAPTA
Add Na
4
BAPTA at a concentration equal to 3 times the Ca
2
þ
concentration in
the medium. Essentially no pH adjustments are necessary. Because of its relative
insensitivity to decreasing pH (
Fig. 8
), BAPTA can be used more conveniently for
scavenging Ca
2
þ
at lower pH values than EGTA.
15
3 times if pH
7.0, 4 times if pH
<
7.0.