Environmental Engineering Reference
In-Depth Information
times higher than the pressure
p
at a given temperature. This violates the neces-
sary condition for the existence of a gas bubble in a liquid (the Eq. (5.16)) and
prevents phase separation, since the external pressure leads to a collapse of gas
bubbles, that is, to its “dissolution” of the pore fluid, if it occurs at all.
It is clear that the required number of methane molecules to form a bubble is
equal to
W
−
27
⋅
== =
⋅
179, 6 10
N
520
−
27
w
0, 345 10
0
where, W
n
- Volumeof the bubble with diameter of 7nm.
The minimum diameter of a bubble of oil in water is calculated based on ap-
proximately similar considerations.
A bubble of oil in an aqueous medium can be considered as an ensemble of
molecules, which are able to form an interface between the liquid phases. The
above (average) radius of the volume of such an ensemble can be obtained from
the Eq. (5.11):
2
s
R
=
(5.18)
pp
π
−
g
n
where,
s
- Border tension coefficient in the water- oil, equal to approximately
,
where,
p
- additional molecular pressure in the equation of van der Waals forc-
es, which for a liquid at condition of it incompressibility it is possible to express
from the of the same equation of van der Waals forces:
kT
�
�
(5.19)
p
=−
+
p
€
‚
n
π
ƒ
w
„
where, w - The real volume of one molecule of the liquid, and“minus” sign due
to the fact that the pressure force directed toward the center of the bubble of oil.
Then from the Eqs. (5.18) and(5.19) we have finally
s
==
+
4
w
d
2
R
kT
(5.20)
n
wp
n
π
The resulting formula determines the minimum value of the diameter of the
bubble of oil in a water medium. In this case the real volume of the hydrocarbon
molecule with one carbon atom-methane, .
The total mass of hydrocarbons that are brought by failution flow per unit area
of contact OGMT-rock reservoir is defined as
t
(5.21)
ò
G =
V dt
G
0
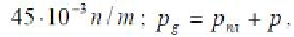
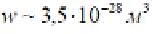




Search WWH ::

Custom Search