Environmental Engineering Reference
In-Depth Information
The molecular energy of the ensemble, keeping a bottle from collapse (i.e.,
from the dissolution of gas), is defined by the van der Waals forces, in which
force of intermolecular interaction can be ignored, since the gas has virtually no
internal pressure. In this case, the magnitude of this energy per unit volume of a
gas bubble is equal to the pressure
p
in the bubble:
0
NkT
p
=
0
(5.13)
WW
−
0
where,
T
- Absolute temperature of thegas °K;
k
- Boltzmann's constant;
; - Volume per one molecule of gas at the critical tempera-
ture and pressure;
w
- the real volume ofagas molecule.
From (5.13) after simple transformations, withtaking into account expression-
for
w
0
we have
pW
N
=
0
kT
+
p w
(5.14)
00
which after substitution inEq. (5.12) gives
kT
p
=
(5.15)
0
Mw
/
r−
0
The condition of the bubbles floating necessarily implies the existence of the
phase boundary, that is, surface bounding the volume of the bubble. A necessary
and sufficient condition for such a boundary is the equality of pressures in gas and
liquid phases:
pp
=−
(5.16)
0
Substituting the values of pressures from Eqs. (5.11) and (5.15) gives the de-
sired diameter of the bubble in the form of
s−r
==
+ −r
4(
Vp
M
/ )
0
d
2
R
kT
(5.17)
(
)
n
p
w
M
/
n
π
0
Calculation shows that the diameter of the molecule the simplest hydrocarbon
gas-methane is equal to 0.38 nm,
If thistake into account, that
then from Eq.
(5.17) is easy to determine the value of the minimum diameter of the gas bubble.
Under hydrostatic pressure of petroleum = 20 Mpa, it is 7 nm. This is
more than twice the diameter of the pore channels. Consequently, in the cramped
conditions of the pore space of OGMT formation of a gas bubble is impossible,
because the capillary pressure in the ducts with a diameter of 3 nm to more than 5

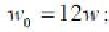
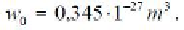
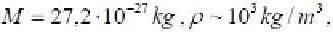





Search WWH ::

Custom Search