Chemistry Reference
In-Depth Information
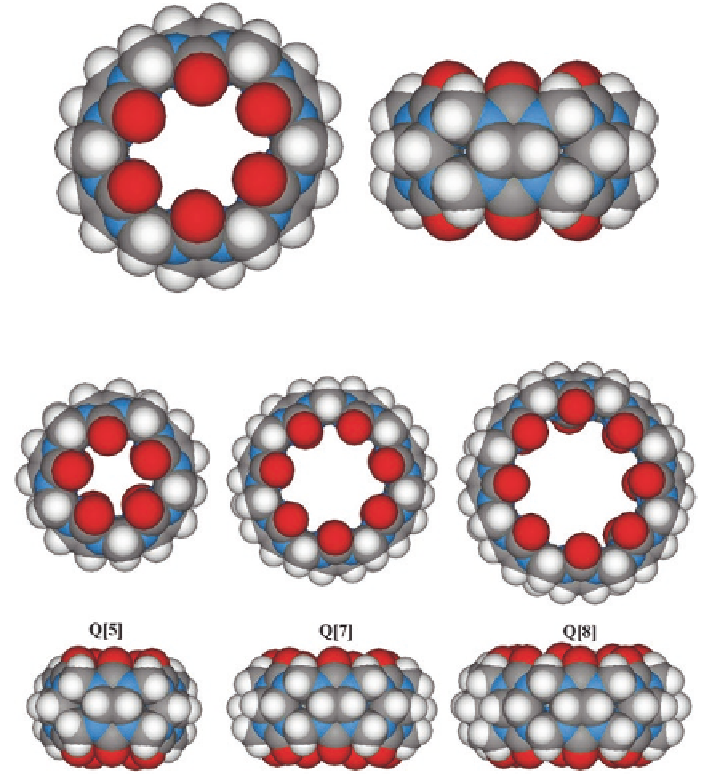
Fig. 1.1
X-ray crystal structure of Q[6]
Fig. 1.2
X-ray crystal structure of Q[5], Q[7], and Q[8]
Less than two years later, Day and coworkers crystallized and identified the
structure of Q[10], which always includes a Q[5] molecule in synthetic processes
(Fig.
1.3
left) [
10
]. Most interesting is our recent discovery of cucurbit[14]uril,
the largest Q[
n
], which has 14 normal glycoluril units linked with 28 methylene
bridges. However, it seems to be formed from 14 units of the glycoluril-(CH
2
)
2
-
moiety with a 180° twist. As a consequence, it does not have a normal cavity like
most cucurbit[
n
]urils, but instead has a folded, figure-of-eight conformation. We
have therefore named it twisted cucurbit[14]uril (tQ[14]) (Fig.
1.3
right) [
11
].
Furthermore, tQ[14] has good solubility in both water (41.0 mmol/L) and organic
solvents such as dimethylsulfoxide (18.0 mmol/L). Overall, these results strongly
deliver some important conclusions: larger homologs of Q[
n
]s (
n
> 10) could be
obtained by the traditional synthetic route.
Search WWH ::

Custom Search