Geology Reference
In-Depth Information
also Box 2.6), or pyroxene may be rimmed by amphibole
owing to reaction with hydrous late-stage melts at lower
temperatures.
1500
Melt
Zoning
In a rock that achieved complete chemical equilibrium
between its phases at a given temperature, all mineral crys-
tals would be homogeneous in composition. Igneous and
metamorphic minerals are, however, quite commonly zoned.
Zoning indicates that intracrystalline diffusion has failed to
keep pace with changing external circumstances. Zoning in
igneous minerals (see plate 2) often reflects chemical
evolution of the melt, with which only the rim of the growing
crystal has maintained equilibrium (Figure 3.1.2). Zoning in
igneous and metamorphic rocks may also be a response to
changing physical conditions (
P
,
T
, etc.).
Initial
melt
Core
1400
1300
Melt +
Plagioclase
ss
Later
melts
Plagioclase
ss
1200
Final melt
Rim
Calcic core
Sodic rim
1100
0
20
40
60
80
100
NaAlSi
3
O
8
Mass %
CaAl
2
Si
2
O
8
Exsolution
perthites (Figure 2.7) and similar textures in pyrox-
enes (plate 3) represent the solid-state decomposition
of a homogeneous crystal into two immiscible phases
(Figure 2.6). Intra-crystalline exsolution lamellae like
these have a very large area of interface with the host
crystal. the mismatch of structure across this interface
generates a large positive interfacial energy, a situation
undoubtedly less stable than equilibrium segregation into
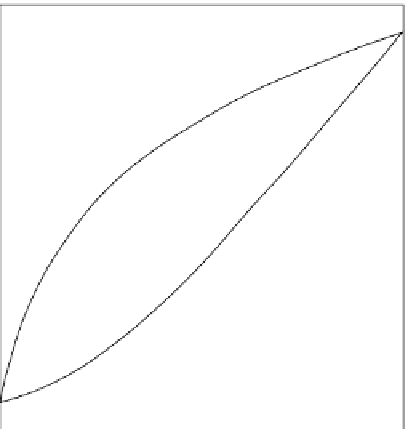
Figure 3.1.2
Formation of a zoned crystal of plagioclase
through cooling and compositional evolution of melt.
separate crystals. the persistence of exsolution lamellae
indicates that diffusion through the crystal was too slow
to allow equilibration.
an example of how exsolution behaviour can be used to
measure cooling rates is given in Box 3.5.
Negative gradient
d
t
d
c
d
c
d
t
Rate = gradient =
Positive gradient
Figure 3.1
Composition-time curves for
reaction 3.1.
t
Rate equation
Because one mole of NO
2
is
produced
for every mole of
NO that is
consumed
by the reaction, the gradients of the
left- and right-hand graphs in Figure 3.1 differ only in
their sign (negative and positive respectively). This dif-
ference is represented by the minus sign in reaction 3.2.
If we repeated the experiment with double the concen-
tration of NO present (
c
O
3
being initially the same as
before), we would find that the initial rate is doubled.




















Search WWH ::

Custom Search