Environmental Engineering Reference
In-Depth Information
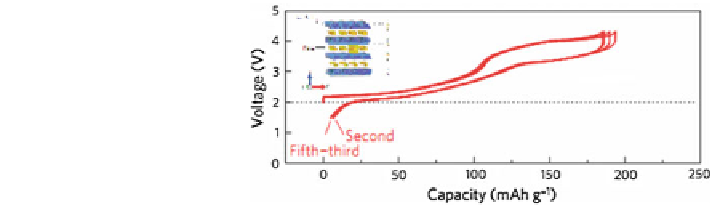
Fig. 13 Galvanostatic
charge/discharge (oxidation/
reduction) curves for Na/P2-
Na
0.67
Fe
0.5
Mn
0.5
O
2
cell at a
rate of 12 mA/g in the
voltage range of 1.5 and
4.3 V [
24
]
stabilities, such as LiFePO
4
, LiMnPO
4
, and so on [
25
,
26
]. Analogously, there are
a wide range of Na polyanion compounds with various structures based on the
difference in the polyanion and the stoichiometry of the elements. In these com-
pounds, the polyanion polyhedra constitute open 3D frameworks to form ion
diffusion channels. Several typical polyanion compounds are illustrated in Fig.
14
.
3.2.1 Phosphate Compounds
Among the various Na polyanion compounds, phosphate-based compounds were
mostly studied for Na-ion intercalation/deintercalation. NASICON Na
3
V
2
(PO
4
)
3
,
as one of the Na super ion conductors, has fast Na-ion diffusion channels in its
crystal structure, [
27
,
32
-
34
] and hence was widely studied. In the NASICON
Na
3
V
2
(PO
4
)
3
structure (Fig.
14
a), the octahedral VO
6
links the tetrahedral PO
4
via
corner to form [V
2
(PO
4
)
3
] unit, which is then interconnected via PO
4
to build up a
three-dimensional framework. Na ions selectively occupy two Na sites (Na1 and
Na2 in Fig.
14
a). One Na ion occupies the Na1 sites, the other two Na ions occupy
2/3 of the Na2 sites. Because the valence of V in Na
3
V
2
(PO
4
)
3
is +3, only two Na
ions can freely move in/out, corresponding to the redox of the V
4+
/V
3+
couple,
thus, the theoretical specific capacity of Na
3
V
2
(PO
4
)
3
is 117.6 mAh g
-1
. Uebou
et al. first reported the Na intercalation behavior of Na
3
V
2
(PO
4
)
3
[
35
]. In order to
improve the electrochemical performance, carbon was used as coating by one-step
solid-state reaction [
33
]. The initial discharge capacity of carbon coated
Na
3
V
2
(PO
4
)
3
reached 93 mAh g
-1
with a voltage plateau at 3.4 V and the
capacity maintained at 90.9 mAh g
-1
after 10 cycles in the voltage range of 2.7-
3.8 V. Kim et al. synthesized highly crystallized, nano-scaled Na
3
V
2
(PO
4
)
3
encapsulated by a conductive carbon-network (Fig.
15
a) by using a polyol-assisted
pyro-synthetic reaction [
32
]. The nanophase Na
3
V
2
(PO
4
)
3
delivered a capacity of
117 mAh g
-1
at 0.08 C (Fig.
15
b). Balaya et al. reported a porous Na
3
V
2
(PO
4
)
3
/C
composite obtained via a soft template approach [
36
]. TEM image (Fig.
16
a)
showed the Na
3
V
2
(PO
4
)
3
nanoparticles were well dispersed in the carbon matrix
(graphene clusters (SP2 type carbon)). This material not only exhibited a high
discharge capacity (116 mAh g
-1
), but also high rate capability (~65 mAh g
-1
at
40 C) (Fig.
16
b). Moreover, nearly 50 % of the initial capacity was retained after
30,000 cycles at 40 C (Fig.
16
c).
Search WWH ::

Custom Search