Environmental Engineering Reference
In-Depth Information
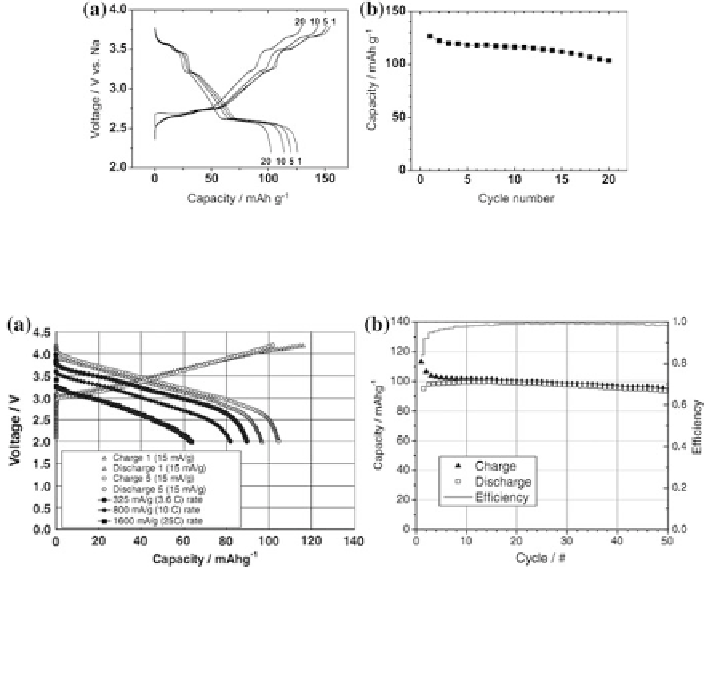
Fig. 11 a Galvanostatic charge and discharge curves and b variation in discharge capacity of
NaNi
0.5
Mn
0.5
O
2
in Na cells at 4.8 mA g
-1
[
21
]
Fig. 12 a voltage profiles (first and fifth charge/discharge cycle; open circles and diamonds) for
Na/Na
0.85
Li
0.17
Ni
0.21
Mn
0.64
O
2
cell between 4.2 and 2.0 V. Additional discharge voltage profiles
for high-rate studies are also shown and labeled in the legend. The trickle charge data points have
been removed for clarity, and b capacity versus cycle number for Na/Na
0.85
Li
0.17
Ni
0.21
Mn
0.64
O
2
cell between 4.2 and 2.0 V [
23
]
Recently, Komaba et al. used Fe to substitute Ni in Na
0.67
Ni
0.5
Mn
0.5
O
2
to
obtain P2-Na
0.67
Fe
0.5
Mn
0.5
O
2
[
24
]. This material achieved a reversible capacity of
190 mAh g
-1
with an average voltage of 2.75 V (Fig.
13
). Hence, the energy
density was estimated to be 520 Wh kg
-1
, which is comparable to that of LiFePO
4
(about 530 Wh kg
-1
) and slightly higher than that of LiMn
2
O
4
(about
450 Wh kg
-1
)[
24
]. If assembled with hard carbon (250-300 mAh g
-1
) or alloy
Sn/Sb (300-500 mAh g
-1
) anode, the energy density of the system would be
hopefully close to that of LiFePO
4
/C battery, even higher than that of LiMn
2
O
4
/C
battery. Besides, this material has the advantage of elemental abundance.
3.2 Polyanion-Based Cathode Materials
Over the last decade, polyanion compounds have attracted much attention as the
cathode materials for Li-ion battery, due to their highly thermal and structural
Search WWH ::

Custom Search