Biology Reference
In-Depth Information
NA
N236
N93
S4'
R126
H
NA
L35
G
NA
K39
NA
S3'
NA
NA
A
NA
NA
C
NA
L41
NA
N174
S2'
D36
C58
C42
W125
I84
L34
I65
L40
L82
E129
K149E
R67
W76
R77A
L73
R74
R75
F
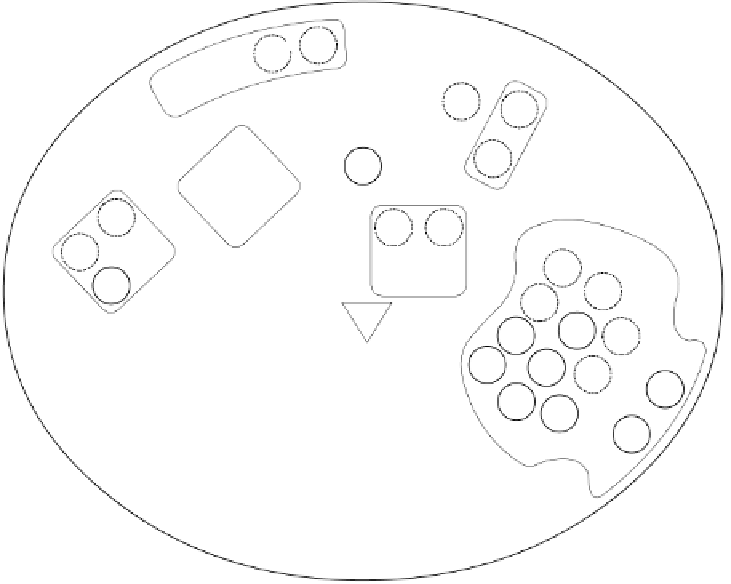
Figure 10.4.
Schematic view of the active site canyon (bold contour) of early mammalian
protein C (Wacey
et al
., 1997). The location of the active site triad residues (His 57, Asp
102, Ser195, chymotrypsin numbering) is denoted by a shaded triangle. Residues which
are conserved between the vitamin K-dependent factor ancestral protein and early
mammalian protein are circled. Nonconserved residues are circled with a broken line. H:
heparin-binding site, G: glycosylation site, C: chemotactic region, A: aryl-binding site, F:
fibrinogen-binding exosite, S
: specificity sites C-terminal to cleavage.
of early mammalian protein C was split along its equatorial axis. The 'North'
patch had acquired three non-conservative mutations, all of which were to neutral
Ile and Leu residues; the resulting changes in both polarity and geometry proba-
bly reflected adaptation to new substrates viz. factors VIIIa and Va. By contrast,
the 'South' patch had not experienced any non-conservative substitutions. With
the exception of Leu73, Arg75, and Asp186A, residues analogous to the fibrino-
gen-binding patch of extant thrombin (Stubbs and Bode, 1993) which are located
outside of the active site were absent from early mammalian protein C. It may be,
therefore, that early mammalian protein C was unable to bind fibrinogen.
An electrostatic view of the early mammalian protein C molecule (
Figure 10.5
,
bottom right) reveals an anionic patch (P4) in the 'South-West' corner of the mol-
ecule. This patch appears to have become moderately expanded in extant human
protein C and comprises (i) residues analogous to the fibrinogen-binding residues
of the 'South' active site, (ii) Lys38, a fibrinogen-binding residue external to the
active site, and (iii) the thrombomodulin-binding residues Lys36 and Arg75 of