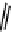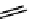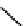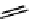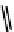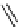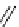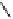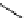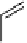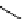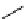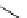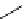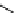Chemistry Reference
In-Depth Information
*CH
3
N
N
CH
3
CH
3
11
CH
3
OTf, RT
O
O
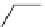
scheme 4.6
N
-[
11
C]methylation of nordoxepin using
11
CH
3
OTf to form [
11
C]doxepin (
10
).
11
CH
3
Br
N
CH
3
NO
2
11
CH
3
NH
2
H
11
CH
3
I
N
CH
3
CH
3
NO
2
scheme 4.7
indirect C-11 methylation reaction of [
11
C]dimethylamine compounds.
O
O
11
CH
3
I
OCH
3
OCH
3
[Pd
2
(dba)
3
]/P(o-tolyl)
3
CuBr/CsF
11
CH
3
Sn(n-C
4
H
9
)
3
scheme 4.8
stille cross-coupling for the formation of
11
C labelling compounds using
11
CH
3
i.
By combining automated loop and sPE (solid phase extraction) methods and using the more reactive [
11
C]methyl triflate
it is possible to improve labelling efficiencies. This has been exemplified for the preparation of [
11
C]doxepin (scheme 4.6)
[46, 47]. such [
11
C]methylation loop reactions have now been applied to the synthesis of [
11
C]carfentanil [48, 49], [
11
C]-L-
[methyl]methionine [50], [
11
C]gefitinib [51], and [
11
C]flumazenil [52].
Although C-11 methylation reactions are generally performed directly, that is, in one step (C-11 methyl precursor plus
target molecule precursor), there are occasionally advantages to labelling in sequential steps or via so-called 'indirect'
methods. indirect routes, which avoid direct reaction with C-11 labelling agents, can be beneficial if there are multiple
methylation locations on the precursor molecule or if there are purification issues and/or difficulties sourcing suitable target
precursors. An example is the labelling of dimethylamine groups that are prevalent in several tracer molecules (
cf.
[
11
C]
doxepin above), which are therefore a valuable target for labelling strategies. Although dimethylamine groups can be
labelled directly with
11
CH
3
i for
11
CH
3
OTf, the indirect method using [
11
C]dimethylamine and bromide precursors has been
demonstrated to improve precursor handling and [
11
C]dimethylamine product purification (scheme 4.7) [53].
Transition metal catalysed cross-coupling reactions that have found wide application in synthetic organic chemistry have
now been applied to radiolabelling protocols. in recent years, palladium-mediated stille- and suzuki-type reactions for
C-
11
CH
3
bond formation have found application for C-11 tracer synthesis. Palladium-mediated stille-type couplings of orga-
nostannanes with [
11
C]methyl iodide have been the most widely investigated of this type. Organostannanes have wide
functional group tolerances and are easily separated from radiolabelled species after reaction using preparative HPLC; how-
ever, there are concerns about their toxicity, which may prohibit these methods for clinical use. One recent study [54]
reported C-11 methylation of a series of alkenyltributylstannanes giving methylated alkenes and the further application of
this method to the synthesis of
11
CH
3
labelled analogues of 1-methylalkene (scheme 4.8).
Under optimised reaction conditions (solvent/base/Pd precursor/phosphine ligand) reactions could be carried out in
5 min. at 60 °C. The order of reagent addition was found to be critical to give reproducible yields and required that C-11
methyl iodide be added to the palladium complex, followed by the stannane precursor. This is presumably to better facilitate
the oxidative addition step of methyl iodide to the palladium catalyst.
The [
11
C]diaryl alkyne M-MTEB ([
11
C]3-methyl-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl] benzonitrile) radioligand, for
imaging metabotropic glutamate receptors (mglur5), has been synthesised via suzuki and stille palladium-mediated cross-
coupling reactions [55] (scheme 4.9). The suzuki method, which uses microwave radiation, was found to give superior rCy
in shorter reaction times over the stille method.